The compound that attacks pyrex glass is
A
XeF2
B
XeF4
C
XeF6
D
Both A and B
Video Solution
Text Solution
The correct Answer is:C
XeF6 can be sublimed at room temperature it is more susceptible to hydrolysis XeF6 melt to give yellow liquid, since it attacks pyrex glass because silica react with XeF6.
2XeF6+3SiO2→3SiF4+2XeO3
|
Similar Questions
Knowledge Check
HIV attacks
AB-CellsBT-cellsCAntigen presenting cellsDT-helpher cellsIn human body ,HIV attacks mainly
AB-lymphocytesBT-lymphocytesCantibodiesDNone of theseHIV attacks
ARBCsBblood plateletsChelper T cellsDB-cells
The principal constituent of pyrex glass is
View SolutionWhich of the position in the following compound is liable to be attacked by an electrophile ?
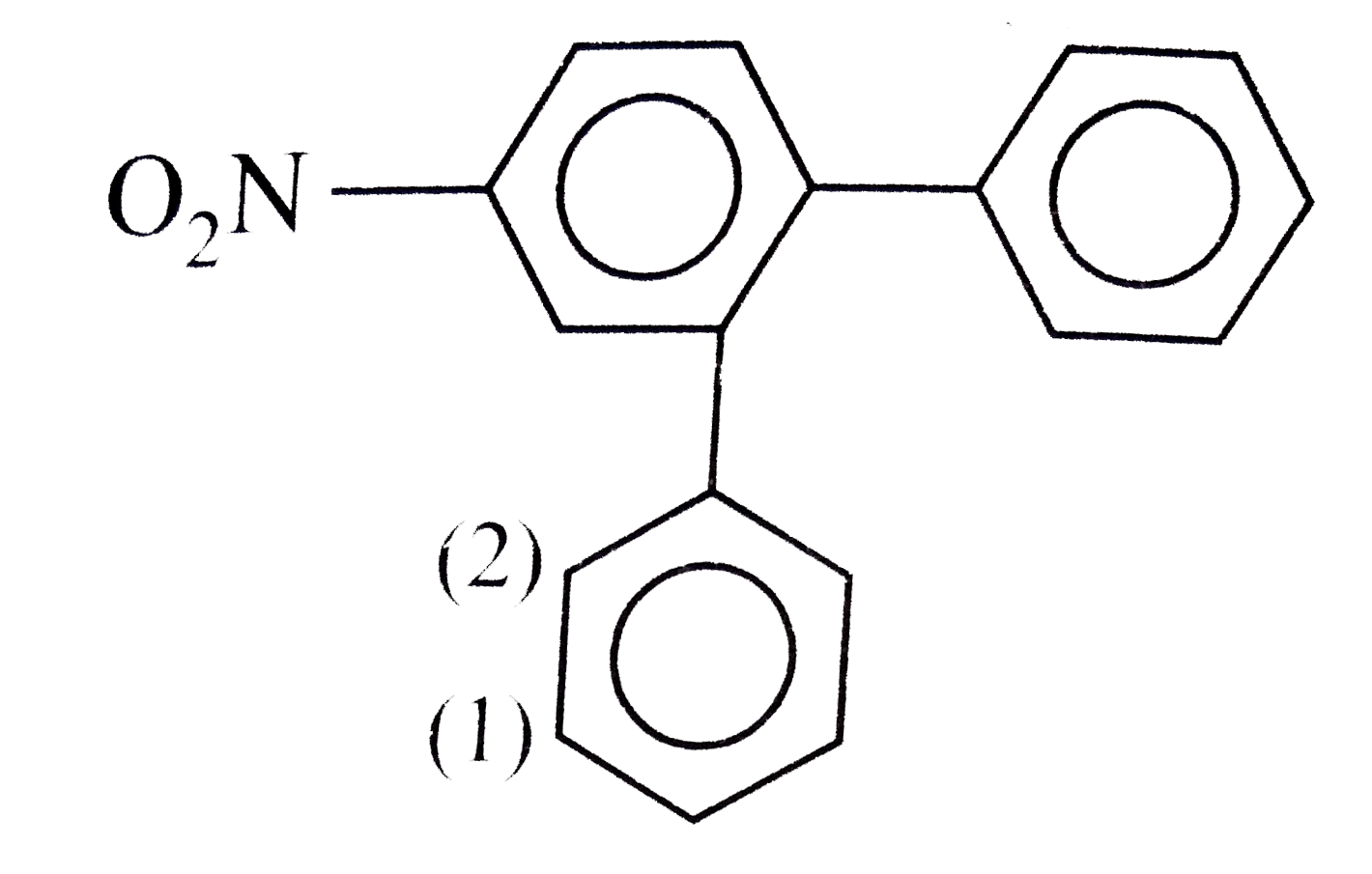
View SolutionWhich of the following is compounds cannot be stored in glass vessels ?
View SolutionStatement -I : Glass is not considered as a true compound
Statement -II : Glass does not have a difinite melting point
View SolutionVenom of cobra attacks
View Solution
