In XeF2.2SbF5
A
Fis forming bridge between Xe and Sb
B
These are two Xe−F bonds with bond length 184± and 235±
C
Both (a) and (b) are correct
D
neither (a) nor (b) is correct
Video Solution
Text Solution
The correct Answer is:C
The following Structure are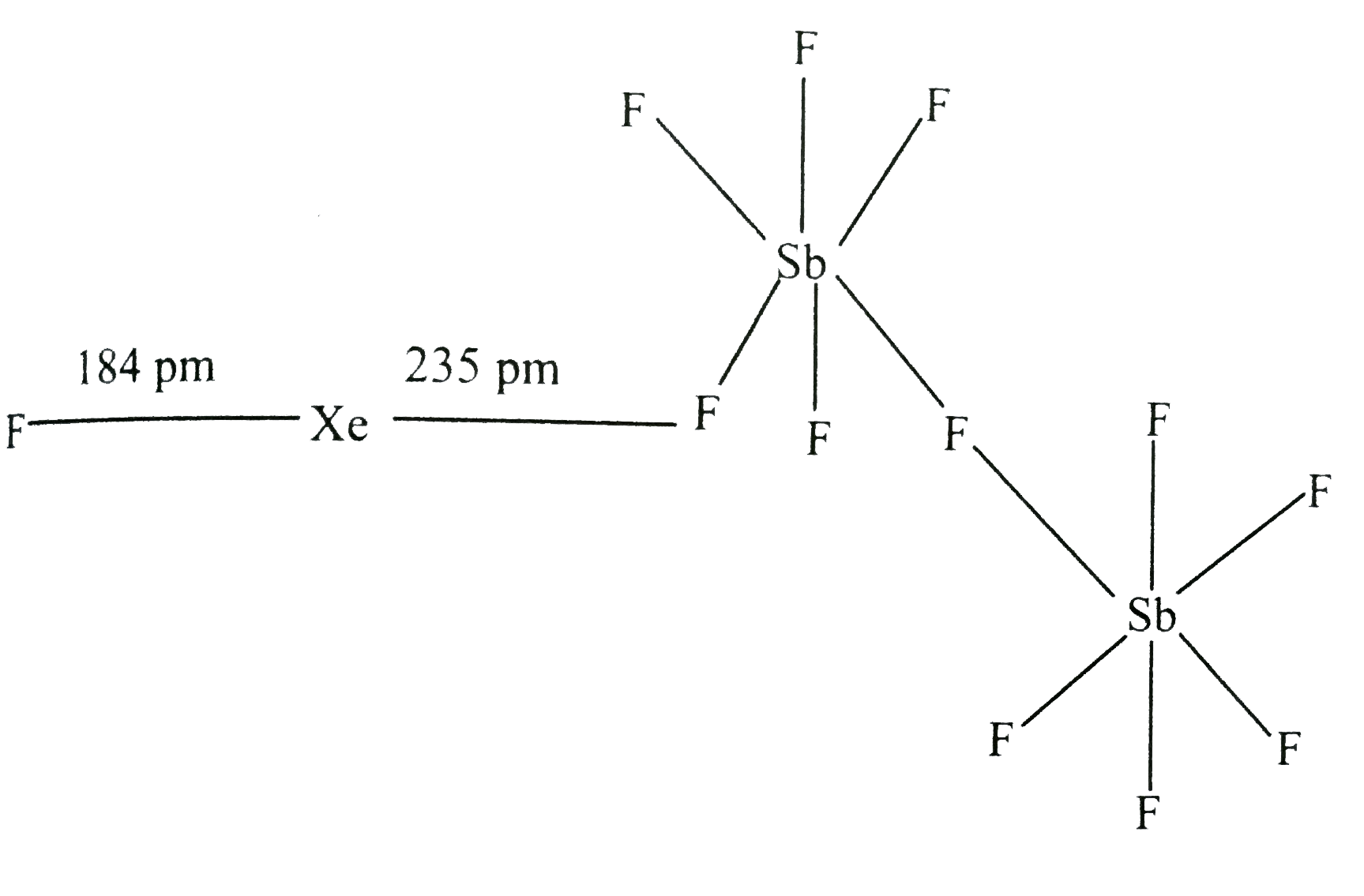
|
Similar Questions
Knowledge Check
(A)+SbF5→[XeF3]+[SbF6]−
Compound (A) isAII and III onlyBI,II and IV onlyCIII and IV onlyDI,II,III and IVIn XeF2,XeF4andXeF6, the number of lonepairson Xe are respectively
A2,3,1B1,2,3C4,1,2D3,2,1In XeF2,XeF4 and XeF6(g) the number of lone pairs on Xe respectively are :
A2, 3, 1B1, 2, 3C4, 1, 2D3, 2, 1
Complete the following reactions :
(i)XeF2+H2→,(ii)XeF6+SiO2→
(iii) XeF6+NH3→ , (iv)XeF6+H2O→
(v)XeF6+SbF5→ (vi)XeF4+4KI→Xe+4KF+2I2
(vii)XeF4+BCl3−78∘C−−−−→
View SolutionComplete the following : XeF2+PF5→
View Solution(a) Draw the molecular structures of :
(i) Peroxodisulphuric acid
(ii) Iodine pentafluoride
(b) Complete and balance the following equations :
(i) NH3+NaOCl→
(ii) XeF4+SbF5→
View SolutionIn XeF2,XeF4 and XeF6 the number of lone pair of Xe is ______ respectively.
View SolutionIn XeF2,XeF4 and XeF6 the number of lone pair of Xe is ______ respectively.
View Solution
