The number of geometrical isomers for [Pt(NH3)2Cl2] is
A
(a) Two
B
(b) One
C
(c) Three
D
(d) Four
Video Solution
Text Solution
The correct Answer is:A
[Pt(NH3)2Cl2]
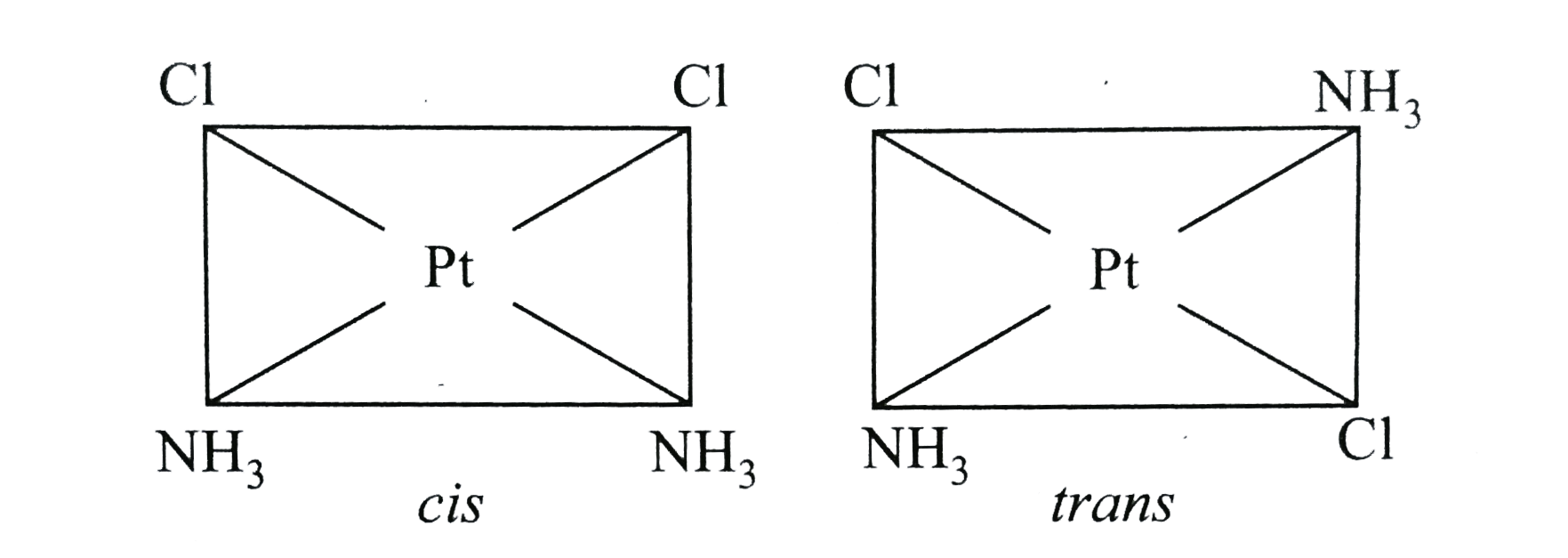
|
Similar Questions
Knowledge Check
The number of geometrical isomers for [P(NH3)2Cl2] is
A2B1C3D4The number of geometrical isomers for [Pt(NH3)2CI2] is
A3B4C1D2The number of geometrical isomers of [Co(NH3)3(NO3)3] are:
A(a) 0B(b) 2C(c) 3D(d) 4
The number of geometrical isomers of [Co(NH3)3(NO2)3] are
View SolutionHow many geometrical isomers arepossible for [Pt(NO2)(NH2OH)(Py)]+?
View SolutionThe number of geometrical isomers for octahedral [Co(NH3)2Cl4]− and square planar [AuCl2Br2]− respectively are :
View SolutionThe number of geometrical isomers for octahedral [Co(NH3)2Cl4]− and square planar [AuCl2Br2]− respectively are :
View SolutionThe number of geometrical isomers for octahedral [Co(NH3)2Cl4]− , square planar [AuCl2Br2]− respectviely are :-
View Solution

