Which is correct in the case of [NiCl4]2− complex?
A
(a) sp3 hybridisation
B
(b) Paramagnetic & Tetrahedral
C
(c) Two unpaired electrons
D
(d) All are correct
Video Solution
Text Solution
The correct Answer is:D
Ni+2 in [NiCl4]2−
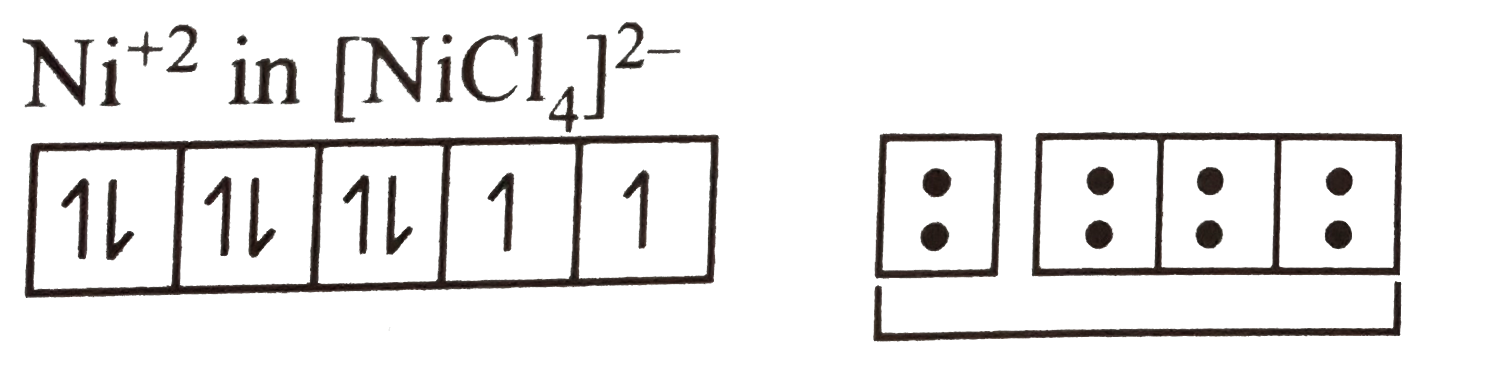
|
Similar Questions
Knowledge Check
Which is correct in the case of [Fe(CN6)]4− complex-
ADiamagneticBOctahedralCd2sp3 hybridisationDAll are correctWhich is true in the case of [Ni(CO)4] complex?
A(a) All are correctB(b) Tetrahedral shape of the moleculeC(c) Hybridization of Ni is sp3D(d) Diamagnetic[NiCl4]2− has geometry
Asquare planarBtetrahedralCsquare bipyramidalDtrigonal bipyramidal
Which is not correct in case of isopentane?
View SolutionWhich is not correct in case of Be and Al ?
View SolutionThe hybrdisation of the complex [NiCl4]−2? is
View SolutionThe magnetic moment (spin only) of [NiCl4]2+ is
View SolutionThe complex []Ni(CN)4]2− is diamagnetic and the complex [NiCl4]2− is paramagnetic . What can you conclude about their molecular geometries ?
View Solution

