AgNO3 does not give precipitate with CHCI3 because .
CHCI3 does not ionise in water
AgNO3 does not reacts with CHCI3
CHCI3 is chemically inert
None of these
The correct Answer is:A
CHCI3+AgNo3→ No react
CHCI3 is a covalent compound It does not ionize in water .
Topper's Solved these Questions
- ORGANIC COMPOUNDS CONTAINING HALOGENSBook:A2ZChapter:ORGANIC COMPOUNDS CONTAINING HALOGENSExercise:Methods Of Preparationof Haloarenes
Explore 19 Videos
- ORGANIC COMPOUNDS CONTAINING HALOGENSBook:A2ZChapter:ORGANIC COMPOUNDS CONTAINING HALOGENSExercise:Chemical Properties Of Haloarenes
Explore 31 Videos
- ORGANIC COMPOUNDS CONTAINING HALOGENSBook:A2ZChapter:ORGANIC COMPOUNDS CONTAINING HALOGENSExercise:Physical Properties And Nuleophilic Substitution Reaction
Explore 34 Videos
- MOCK TESTBook:A2ZChapter:MOCK TESTExercise:Mock Test 2
Explore 45 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGENBook:A2ZChapter:ORGANIC COMPOUNDS CONTAINING NITROGENExercise:Section D - Chapter End Test
Explore 30 Videos
Similar Questions
In the above process product A is
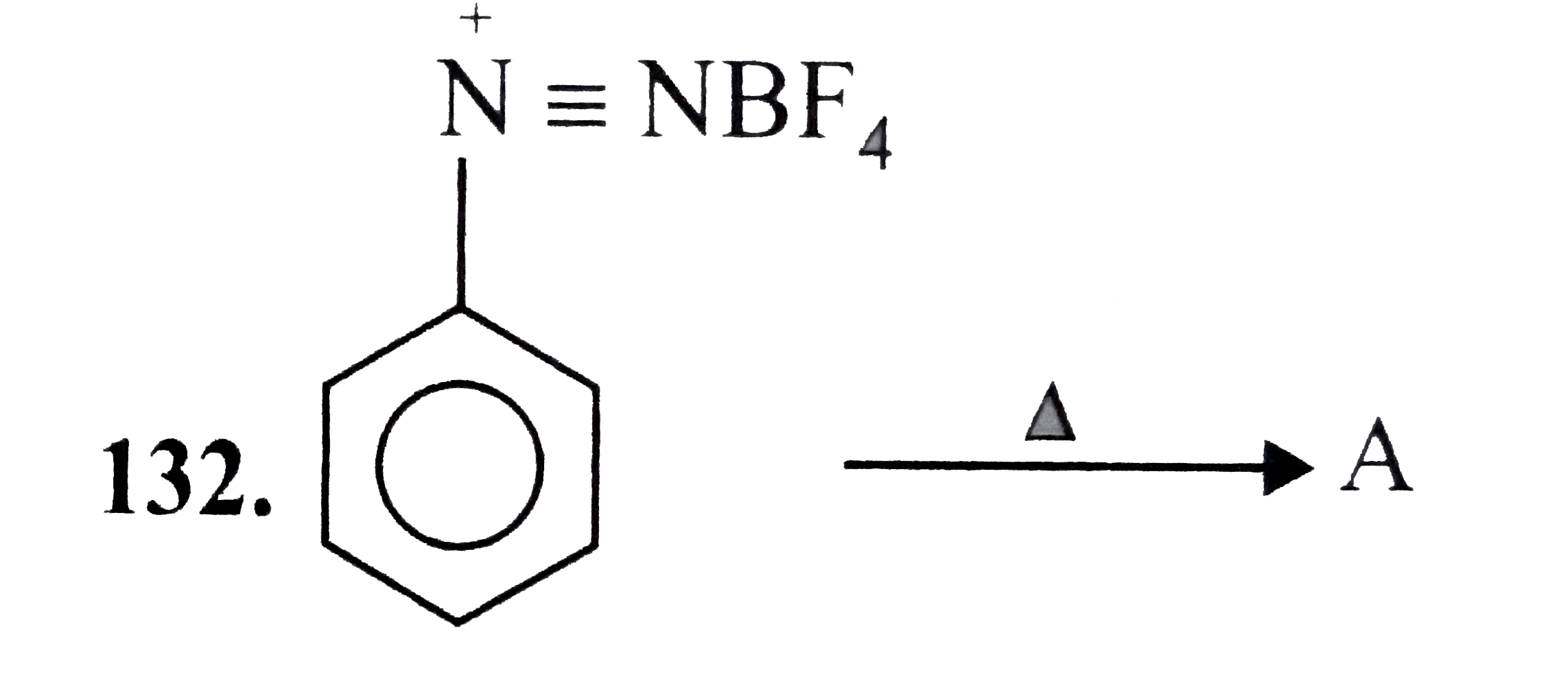 .
.
View SolutionAn aromatic compound of molecular formula C6H4Br2 was nitreated then three isomers of formual C6H3Br2NO2 were obtained The original compound is .
View SolutionFor CH3Br+OH→CH3OH+Br
the rate of reaction is given by the expression .
View SolutionWhat is the density of Na2O having antifluorite-type crystgal stryctutre, if the edge length of cube is 100gm and what is the effect on density by 0.05% Frenkel defect?
View SolutionWhich of the following nitro compounds will show tautomerism?
View SolutionThe halide which does not give a precipitate with AgNO_(3) is
View SolutionCHCl3 does not give white ppt. with AgNO3 because it:
View SolutionWhich of the following does not give a precipitate with alcoholic AgNO_(3) ?
View SolutionWhich one of the following does not give a precipitate with AgNO_(3) solution ?
View SolutionThe following does not give a precipitate either with AgNO_(3) or BaCL_(2)
View SolutionAgNO3 does not give precipitate with CHCI3 becauses ,
View SolutionNaCl_((aq)) gives a white precipitate with AgNO_(3) solution but C Cl_(4) or CHCl_(3) does not , because
View SolutionAgNO3 gives red precipitate with
View SolutionIodoform gives a precipitate with AgNO_(3) on heating but chloroform does not because :
View SolutionThe complex compound which does not give precipitate with AgNO3 solution is
View Solution
