Assertion: Alkyl halides form alkenes when heated above 300∘C
Reason Ch3CH2 I reacts slowly with strong base when compared to CD3CH2 i.
If both assertion and reason are true and the reasone is the correct explanation of the assertion .
If both assertion and reason are true but reason is not the correct explanation of the assertion .
If assertion is true but reasone is false
If assertion is false but reason is true .
The correct Answer is:C
CH3CH2 I reacts more rapidly with strong base in comparison to CD3CH2 I the elimination of HI (or DI) in presence of strong base shown E2 elimination The rate determining step involves the breaking up of C−H (or C-D) bond the C−D bond being stronger than C−H and thus elimination is faster in case of CH3CH2 I .
Topper's Solved these Questions
- ORGANIC COMPOUNDS CONTAINING HALOGENSBook:A2ZChapter:ORGANIC COMPOUNDS CONTAINING HALOGENSExercise:Assertion-Reasoning Questions
Explore 9 Videos
- MOCK TESTBook:A2ZChapter:MOCK TESTExercise:Mock Test 2
Explore 45 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGENBook:A2ZChapter:ORGANIC COMPOUNDS CONTAINING NITROGENExercise:Section D - Chapter End Test
Explore 30 Videos
Similar Questions
Knowledge Check
CH3−NC is formed when CH3−Cl reacts with
AKCNBAgCNCCH3CHODKOH and CH3NH2When (CH3)3CCH2Cl is heated at 300∘C, it gives
A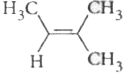 B
B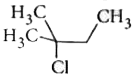 C
C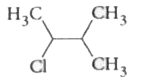 D
D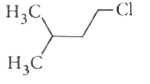
Which is formed when (CH3)NOH is heated?
ACH3NH2BCH3CH2NH2C(CH3)3ND(CH3)2NH
Arrange the given alkyl halides in the order of decreasing rate of dehydrohalogenation reaction i.e., when heated in presence of alc. KOH
CH3−CH2−Cl,CH3−CH2−Br,CH3−CH2−I
View SolutionWhich alkyl halide from the following pair is (i) Chiral and (ii) undergoes SN1 reaction faster ?
(a) (CH3)3CBr
(b) CH3CH2CHBrCH3
View SolutionWhen H2C=CH−O−CH2CH3 reacts with one molre of HI, one of the product formed is ,
View SolutionWhen H2C=CH−O−CH2CH3 reacts with one molre of HI, one of the product formed is ,
View SolutionDehydrohalogenation involves removal of the halogenn atom together with a hydrogen atom from carbon adjacent to the one with halogen atom. Alcoholic KOH is used for dehydrohalogenation. According to saytzeff's rule, when two alkenes may be formed, the alkene which is most substituted is the major product.
Q. Arrange the following alkyl halides in decreasing order of the rate of β-elimination reaction with alcoholik KOH.
CH3CH2CH2Br(i)CH3−C∣CH3H−CH2Br(ii)CH3−CH2−C∣BrH−CH3(iii)
View Solution
