The compound with the lowest boiling point, that is, the most valatile compound is
A
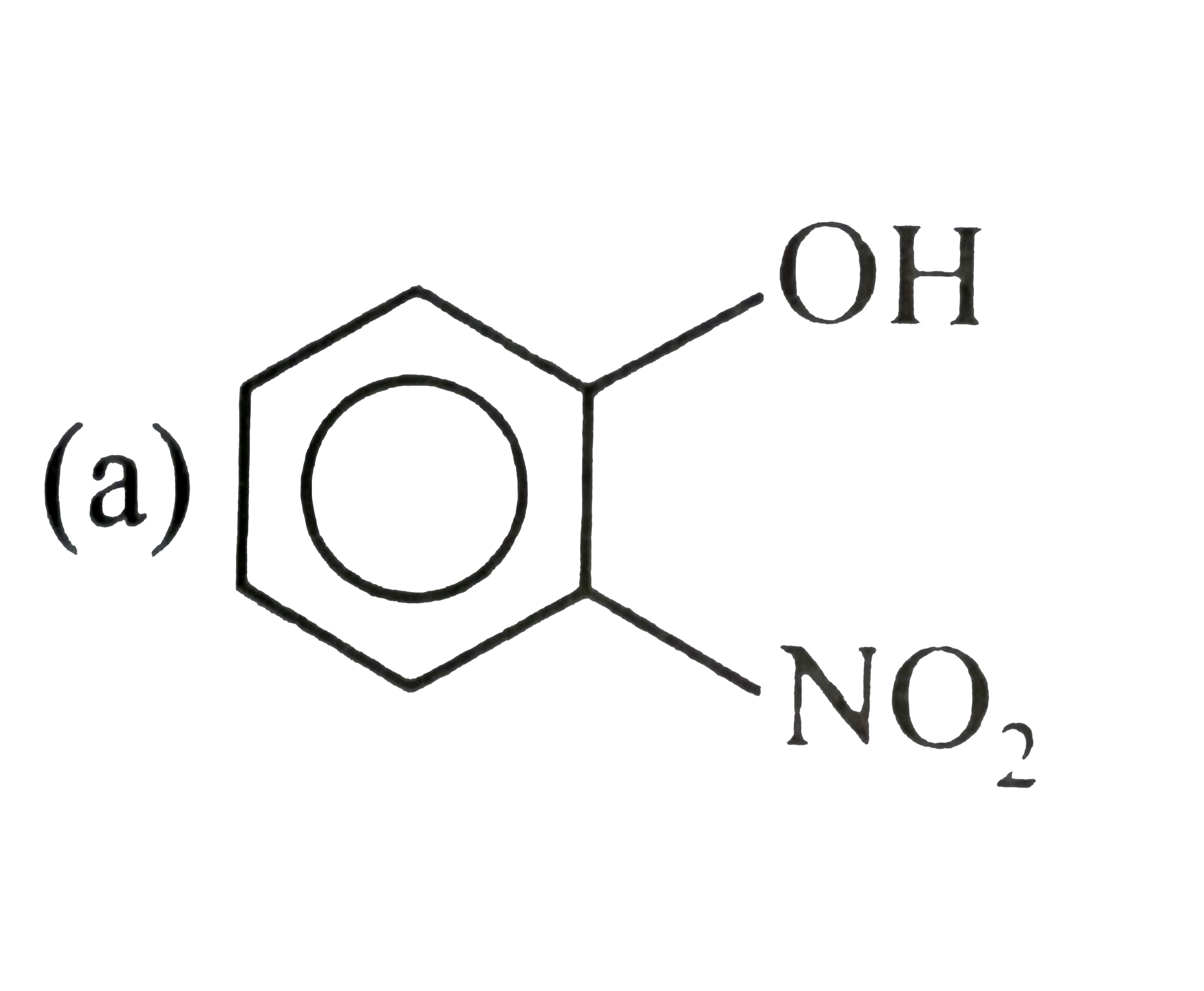
B
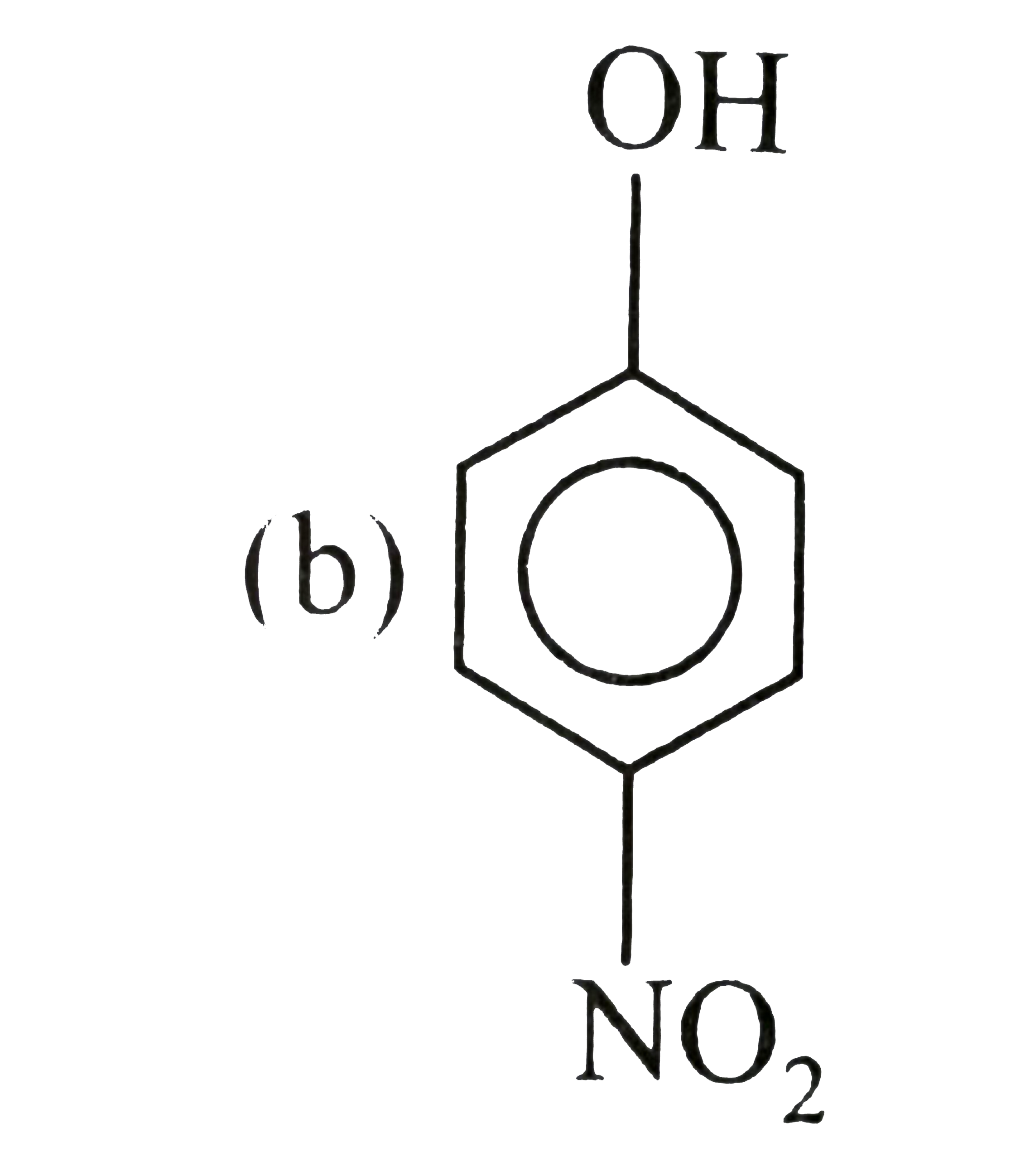
C
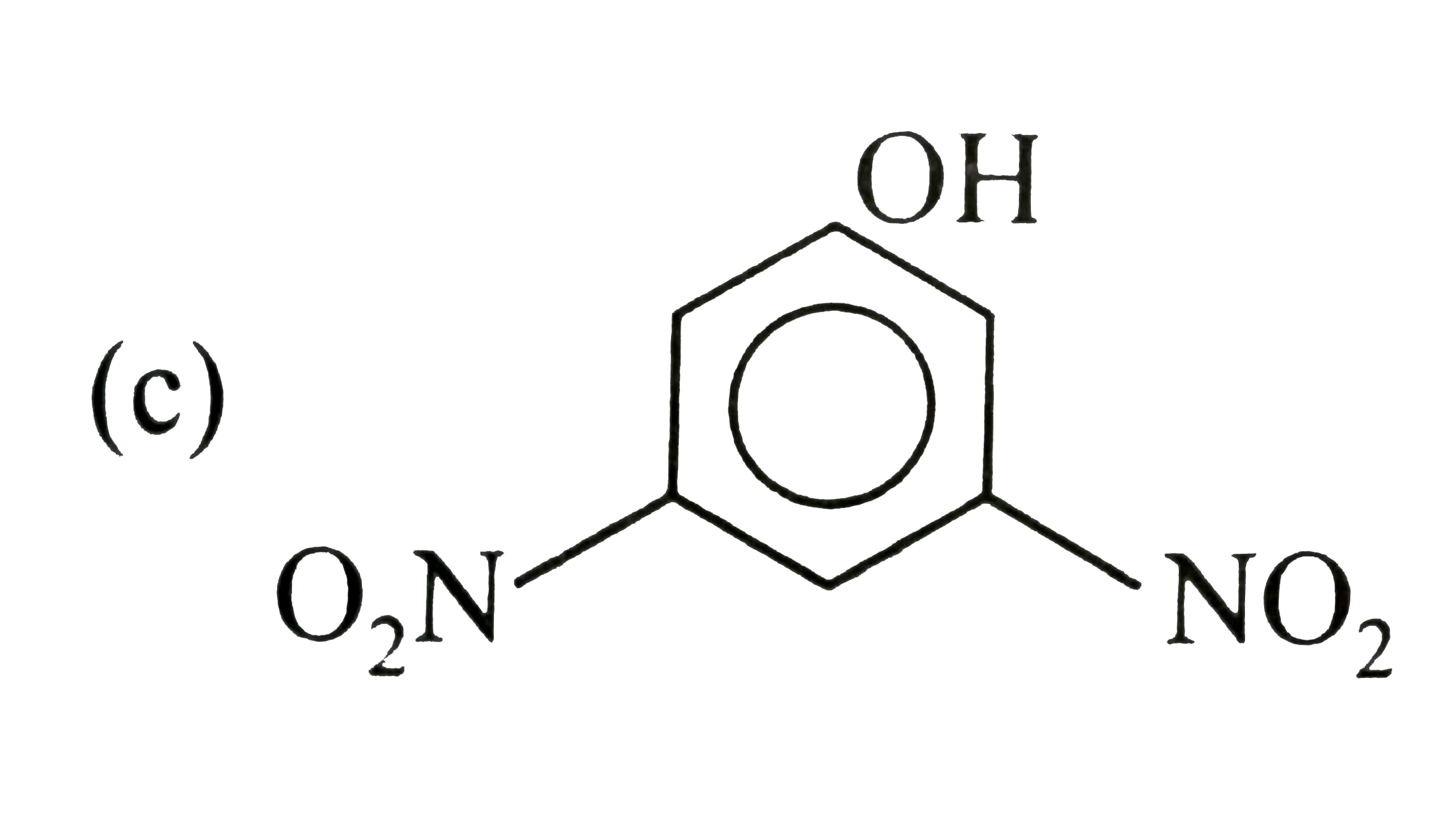
D
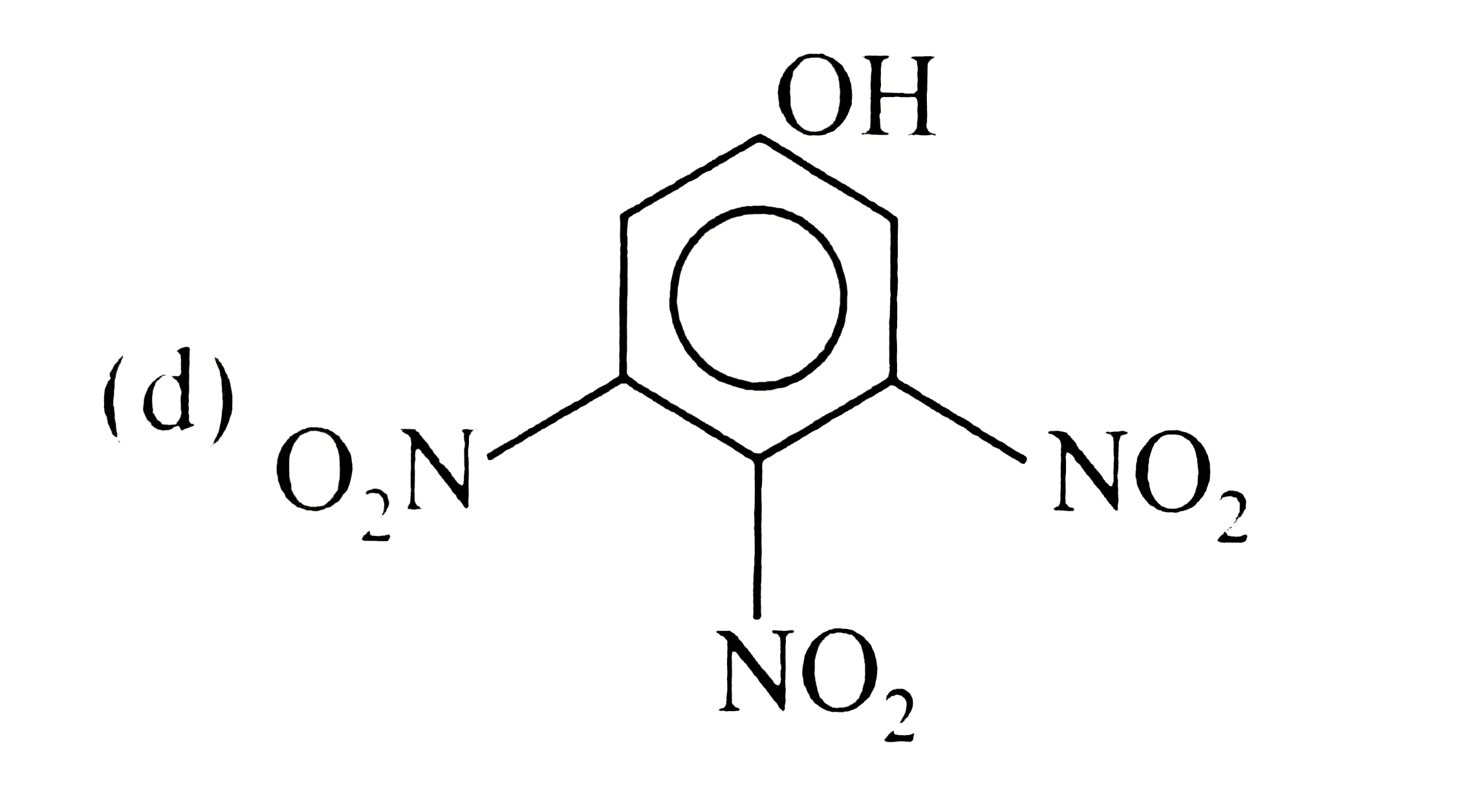
Video Solution
Text Solution
The correct Answer is:A
Phenols capable of forming intermolecular hydrogen bonding have a high boiling point. But (a) has intramolecular rather than intermolecular H-bonding and is the most volatile ocmpound.
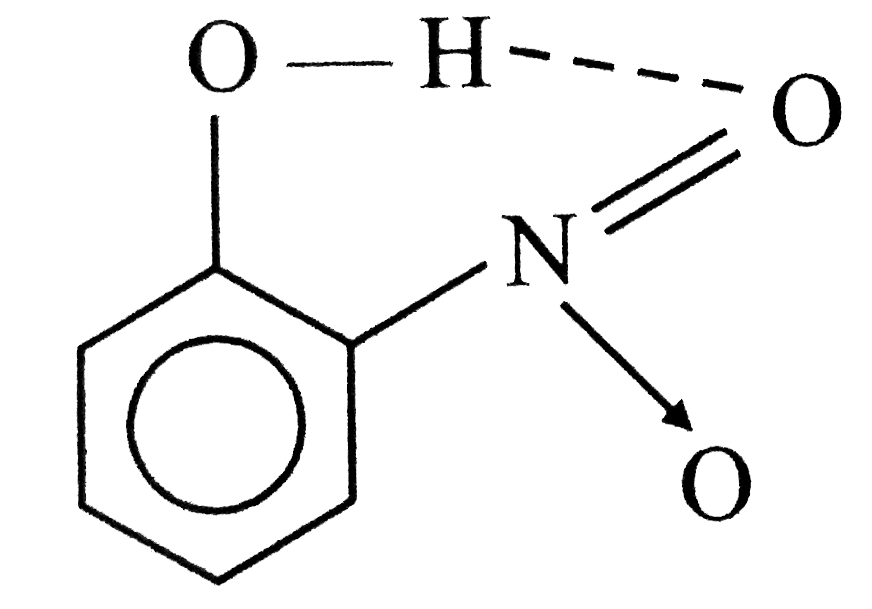
|
Similar Questions
Knowledge Check
The compound which has the lowest boiling point is :
AH2OBC2H5OHCCH3OHDC2H5OC2H5The compound with the highest boiling point is
A(a) n-PentaneB(b) n-HexaneC(c) 2-Methyl butaneD(d) 2,2-Dimethyl propaneThe compound with the highest boiling point is:
A(a) n-PentaneB(b) n-HexaneC(c) 2-Methyl butaneD(d) 2,2-Dimethyl propane

