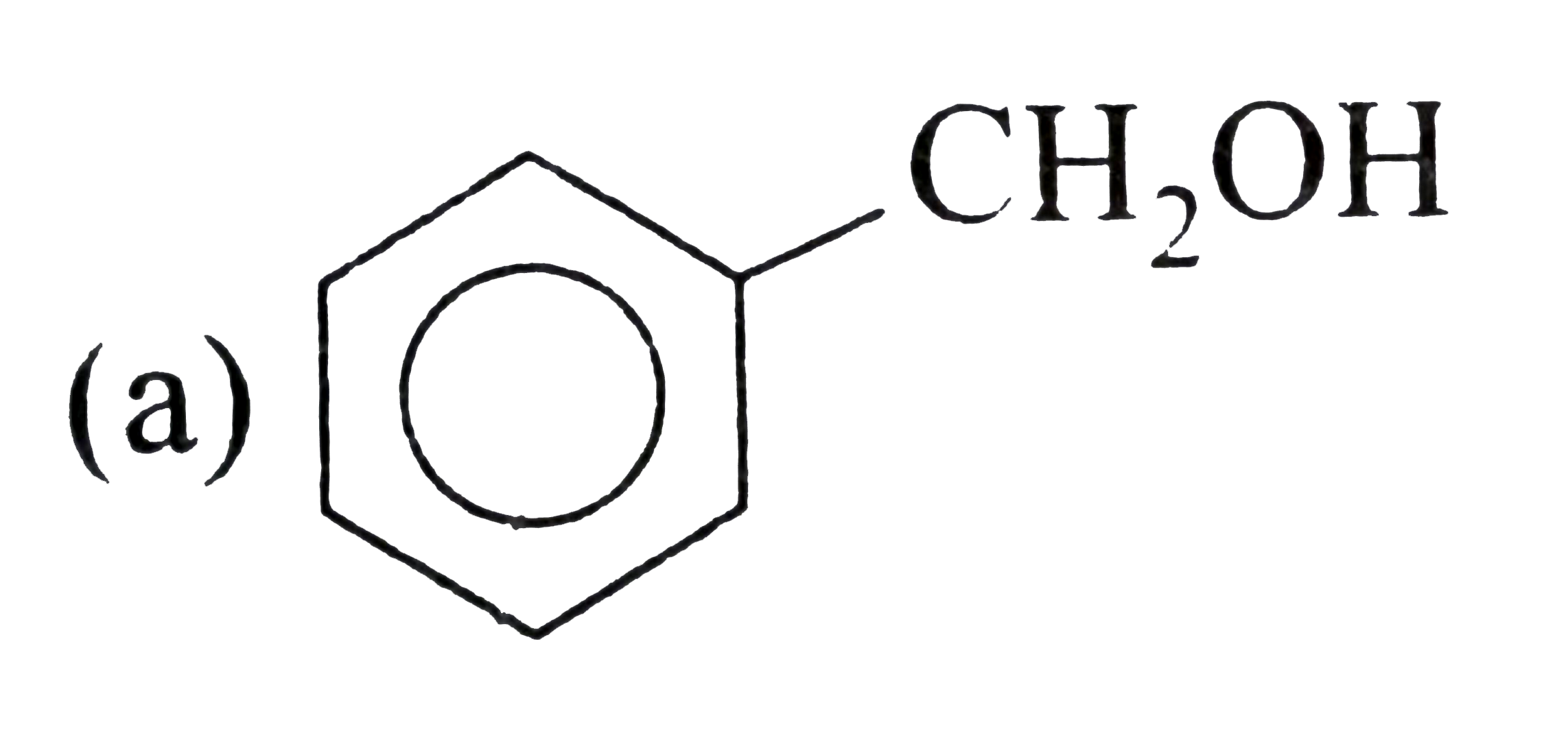
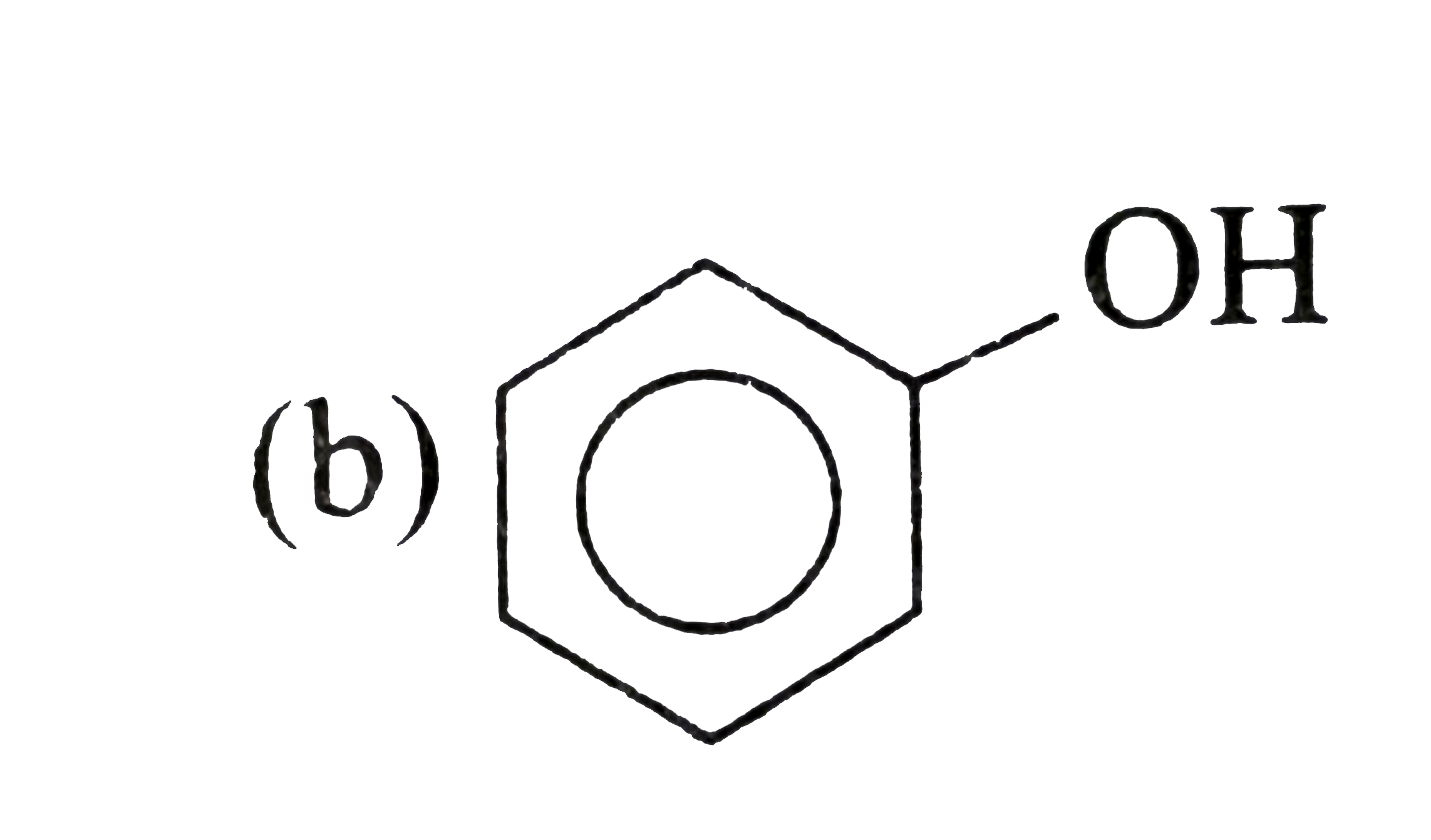
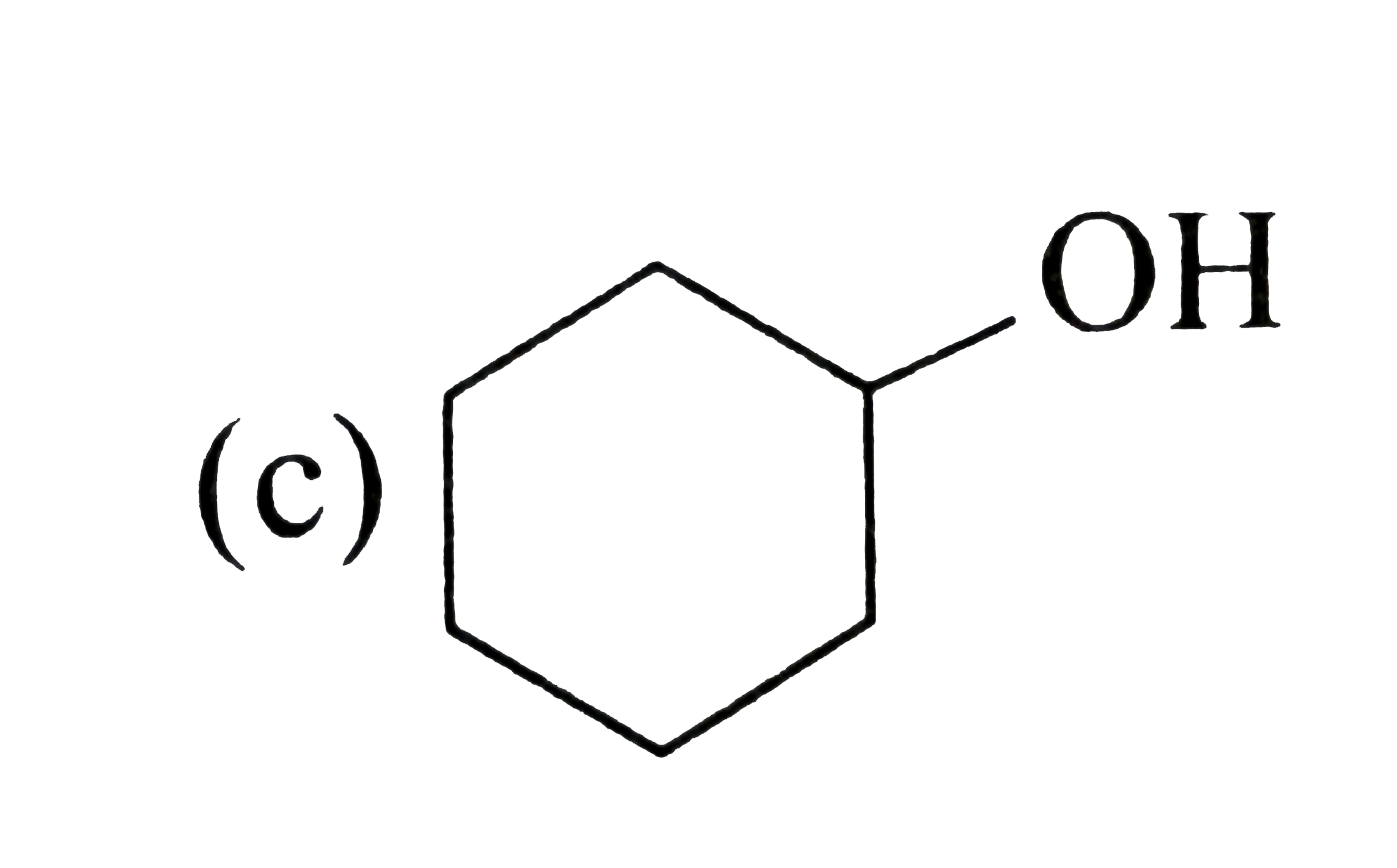
Which one of the following compounds has the most acidic nature?
A
B
C
D
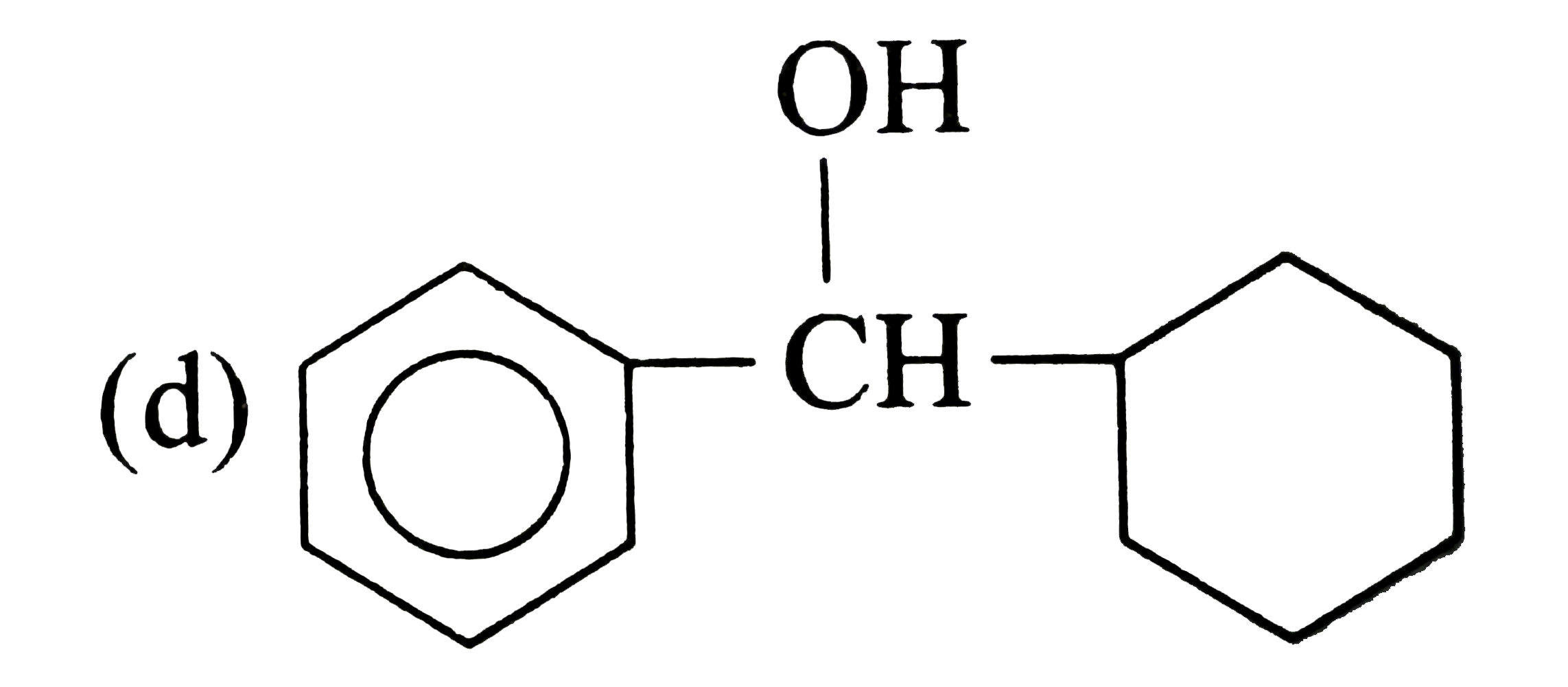
Video Solution
Text Solution
The correct Answer is:B
Phenyl is an electron withdrawing substituent while −CH3 is an electron releasing sunstituent. Moreover, phenoxide ion is more resonance stabilished as compared to benzy1 oxide ion, thus release proton more easily. Thus strong acid among the given options.
|
Similar Questions
Which one of the following compounds has the most acidic nature ?
View SolutionWhich one of the following compounds has the most acidic nature?
View SolutionWhich one of the following compounds has the most acidic nature ?
View Solutionनिम्नलिखित यौगिकों में से किसका अम्लीय स्वभाव सर्वाधिक है ?
View SolutionWhich one of the following compounds has the most acidic nature ?
View SolutionWhich of the following compounds has the most acidic nature?
View SolutionWhich of the following compounds has the most acidic nature
View SolutionWhich one of the following compounds has the most acidic nature ?
View Solution
