SiCL4+2MgCl→X+2MgCl2
'X' on hydrolysis followed by polymerization gives
A
Linear solution
B
Crosslinked silicone
C
Dimer
D
Zerolite
Video Solution
Text Solution
The correct Answer is:A
SiCl4+2RMgCl→R2SiCl2+2MgCl2
R2SiCl2H+2O−−−→R2Si(OH)2

|
Similar Questions
Knowledge Check
Me2SiCl2 on hydrolysis will produce
AMe2Si(OH)2BMe2SiOC−O−⎛⎜ ⎜⎝Me∣Si−O∣ME⎞⎟ ⎟⎠n−∣Si∣−DMe2SiCl(OH)(me)2SiCl2 on hydrolysis will produces:
A(Me)2Si(OH)2B−[O−(Me)2Si−O−]nC(Me)2Si=ODMe2SiCl(OH)(Me)2SiCl2 on hydrolysis will produce.
A(Me)2Si(OH)2B(Me)2Si=OC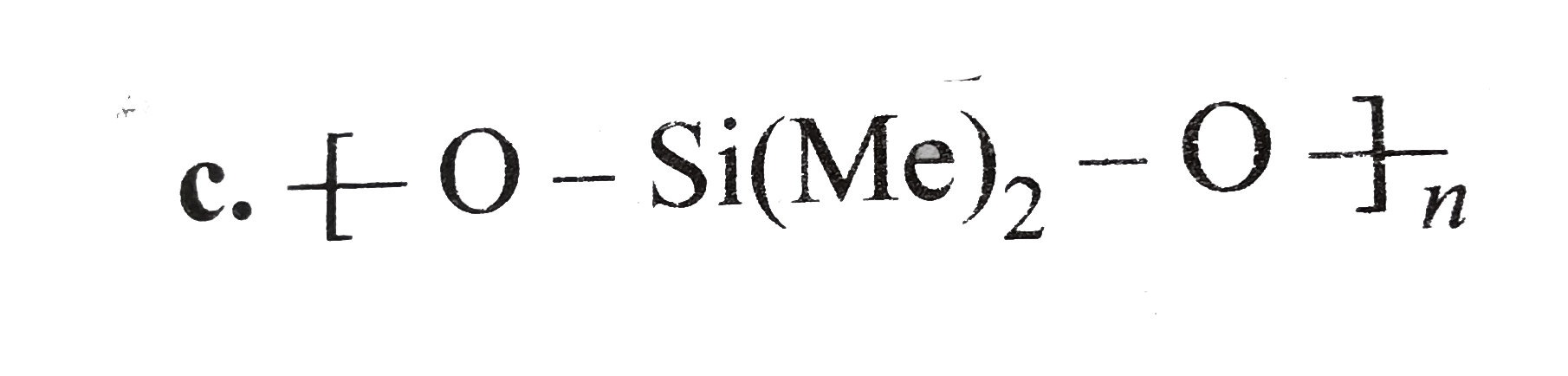 .DMe2SiCl(OH)
.DMe2SiCl(OH)
(CH3)2SiCl2 on hydrolysis and on subsequent polymerisation will produce :
View SolutionSiCl4 on hydrolysis gives:
View SolutionStatement-I: (CH3)3 SiCl produces polymeric silicons on hydrolysis and followed by condensation
Statement-II: Silicon does not form Si=0 due to less effective lateral overlapping of 3p-2p orbitals
View SolutionHydrolysis of dimethyldichloro silane , (CH3)2SiCl2 followed by condensation polymerisation yields straight chain polymer of
View SolutionXeF2 on complete hydrolysis gives:
View Solution

