The electronegativity of H and CI are 2.1 and 3.0 respectively. The correct statement (s) about the nature of HCI is/are:
A
17% ionic
B
83% ionic
C
50% ionic
D
100% ionic
Video Solution
Text Solution
The correct Answer is:a
% ionic character =16(XA−XB)+3.5(XA−XB)2
16(3.0−2.1)+3.5(3.0−2.1)2
=14.4+2.835=17.235≈17%
|
Similar Questions
Knowledge Check
The correct statement (s) about starach
AIt is a pure single compoundBIt is mixture of two polysacchardes of glucoseCIt involves the (C1−C4)α− glucosidic linkage between two α−D glucose units.DIt involves branching by (C1−C6) glycosidic linkage.The correct statement (s) about oxygen is/are
Ait is paramagnetic in natureBit has three stable isotopesCit exhibits allotropyDAll of the aboveCorrect statements (s) about
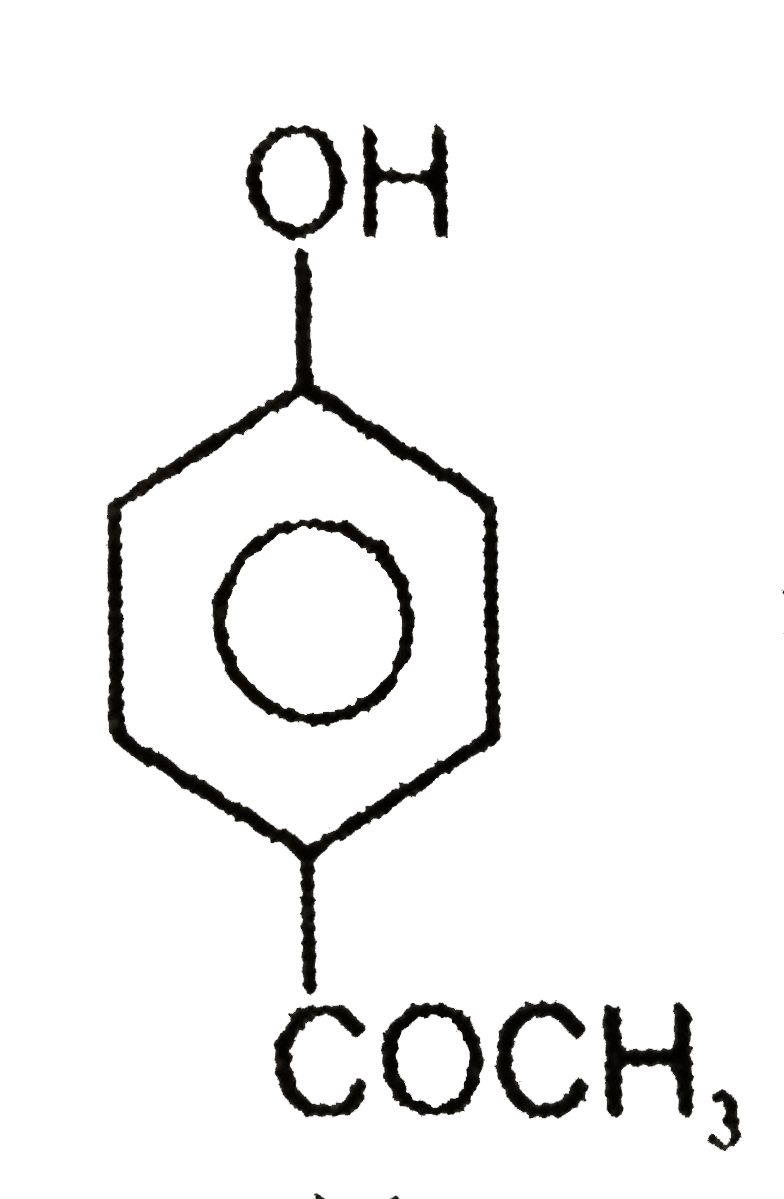 is /areAIt gives coloured solution with neutral FeCl3 solutionBIt liberates H2 gas with Na metalCIt gives positive iodoform test.DIt forms sweet smelling compound with alcohols.
is /areAIt gives coloured solution with neutral FeCl3 solutionBIt liberates H2 gas with Na metalCIt gives positive iodoform test.DIt forms sweet smelling compound with alcohols.

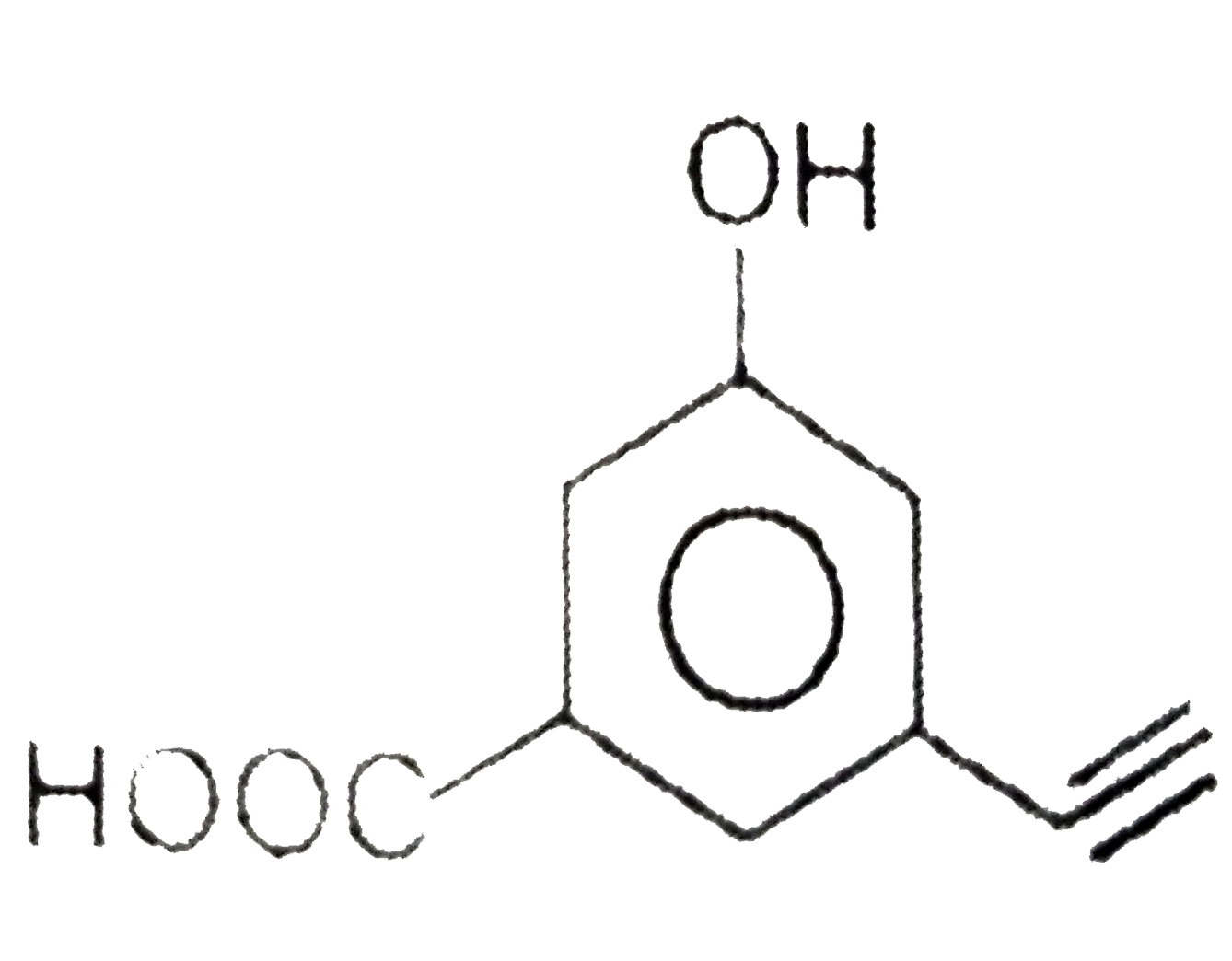 is /are
is /are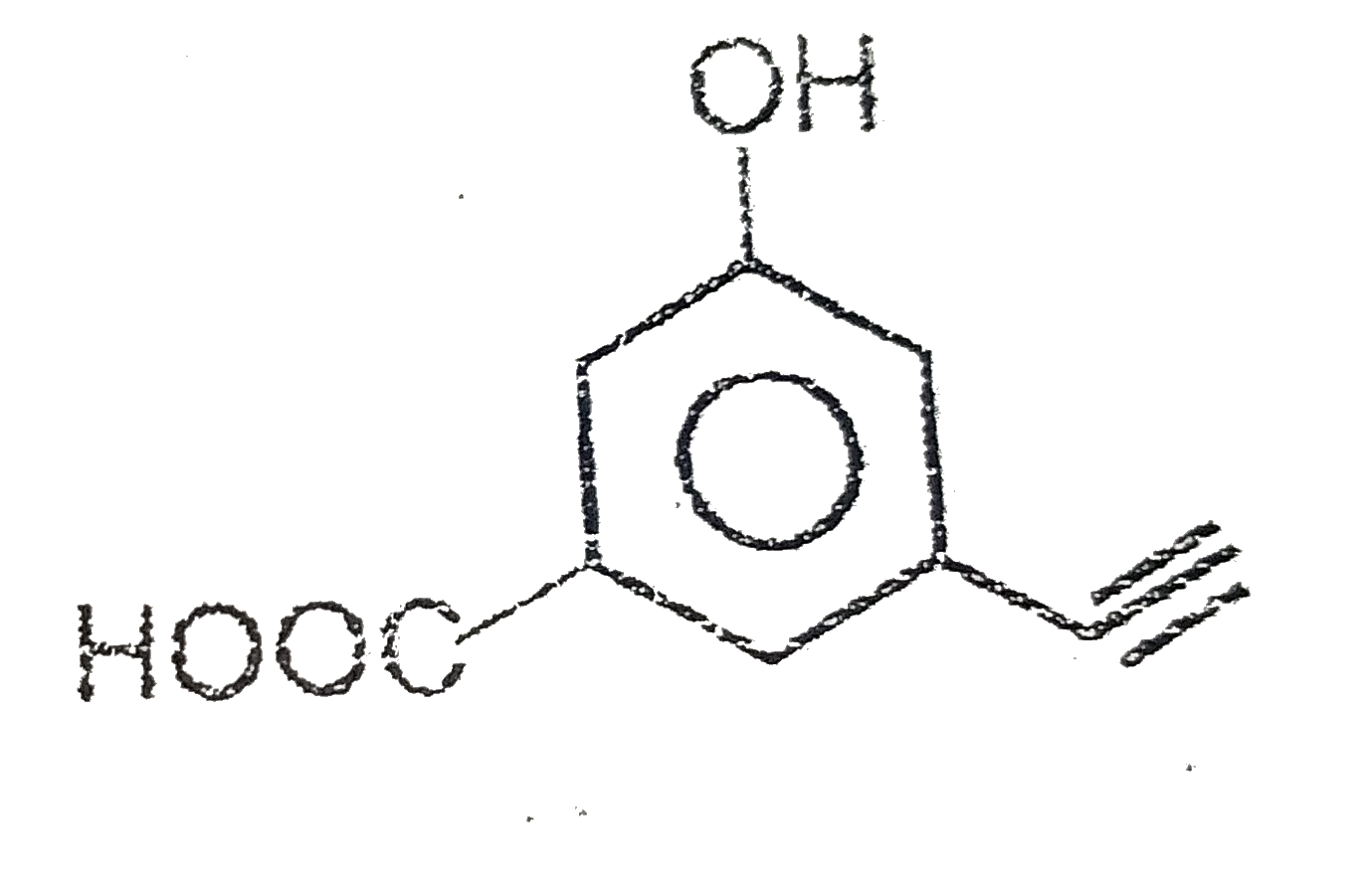 is
is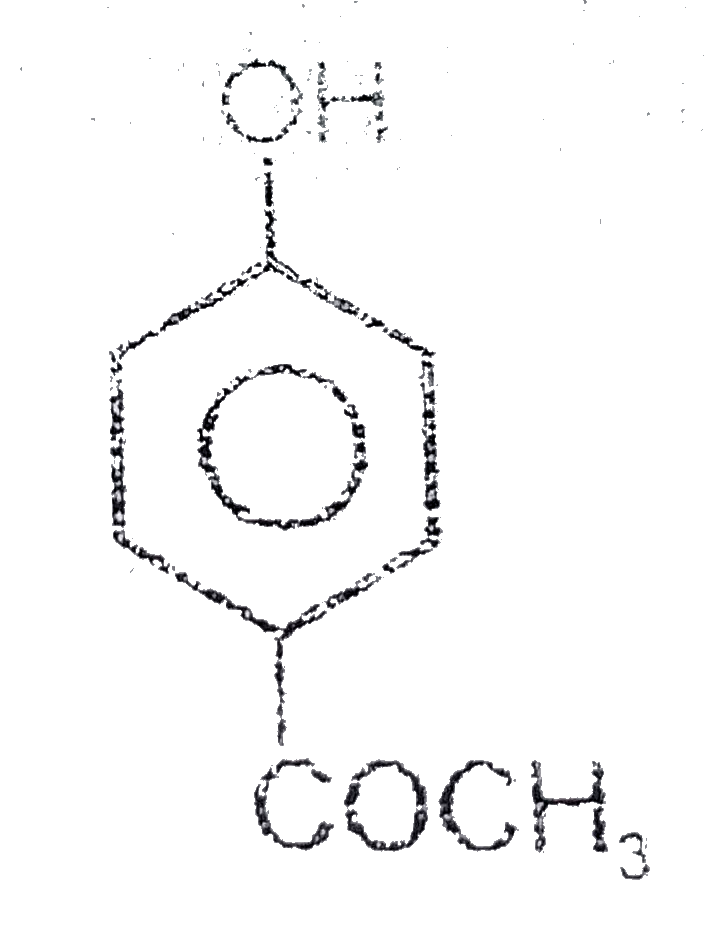 is
is