Main axis of diatometic molecule is z, molecular orbatals pxandpy overlap to form, which of the following orbital?
π- molecular orbital
σ- molecular orbital
δ- molecular orbital
no bond will form
The correct Answer is:A
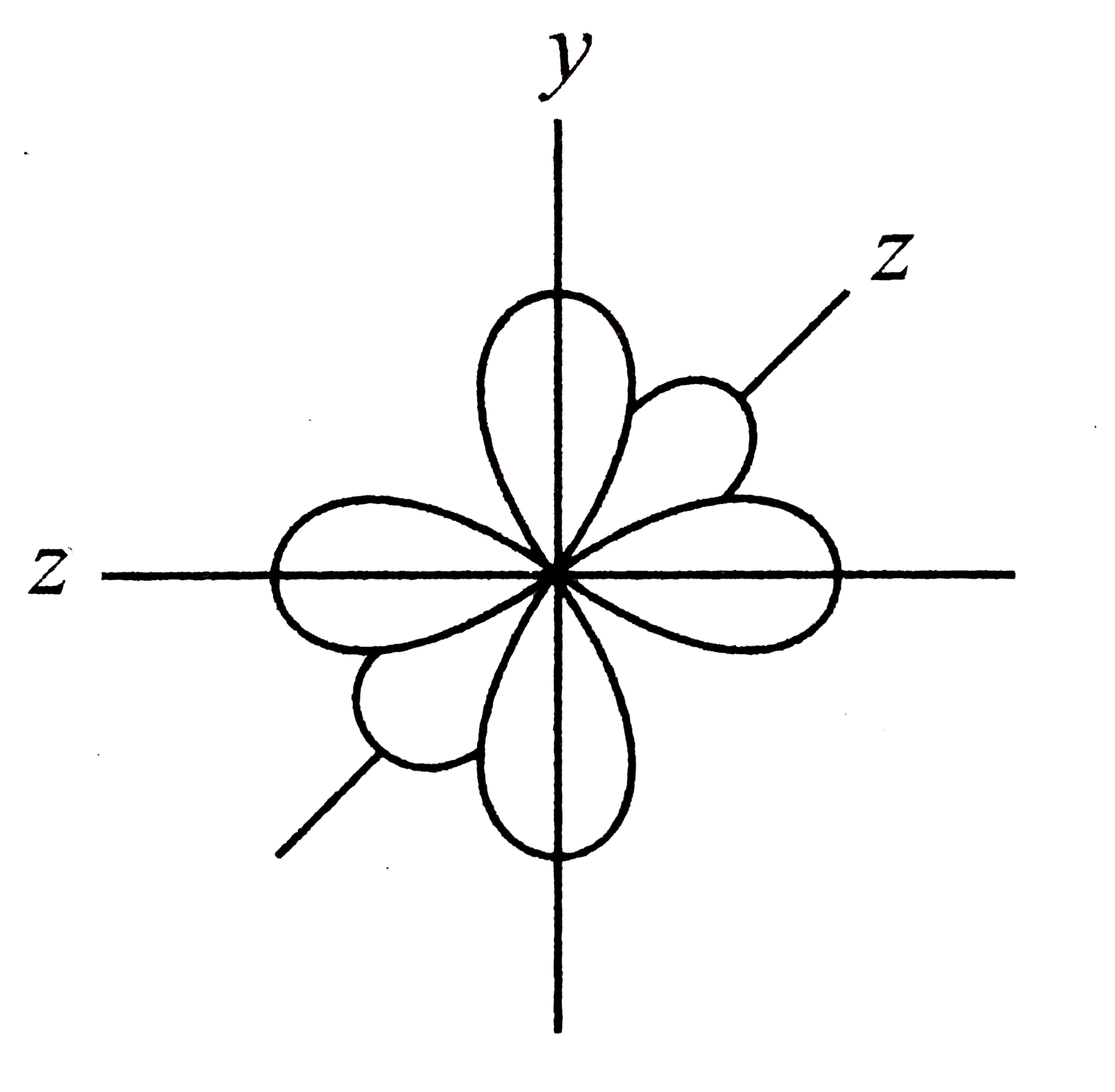
PyandPz overlap laterally forming π bonds
Similar Questions
Knowledge Check
Main axis of diatometic molecule is z, molecular orbatals pxandpy overlap to form, which of the following orbital?
Aπ molecular orbitalBσ molecular orbitalCδ molecular orbitalDNo bond will formThe main axis of diatomic molecule is z. The orbitals px and py overlap to form
Aπ molecular orbitalBσ molecular orbitalCδ molecular orbitalDno bond will be formedIf Z−aξs is the molecular axis, then π−molecular orbitals are formation by the formed by the overlap of
As+pzBpx+pyCpz+pzDpx+px
In which of the following molecules, σ2pz molecular orbital is filled after π2pxandπ2py molecular orbitals ?
View SolutionComprehension given below is followed by some multiple choice question, Each question has one correct options. Choose the correct option.
Molecular orbitals are formed by the overlap of atomic orbitals. Two atomic orbitals combine to form two molecular orbitals called bonding molecular orbital (BMO) and anti-bonding molecular orbital (ABMO). Energy of anti-bonding orbital is raised above the parent atomic orbitals that have combined and hte energy of the bonding orbital is lowered than the parent atomic orbitals.
energies of various molecular orbitals for elements hydrogen to nitrogen increase in the order
σ1s<σ⋆1s<σ⋆2s<((π2px)=(π2py))<σ2pz<(π⋆2px=π⋆2py)<σ⋆2pz and For oxygen and fluorine order of enregy of molecules orbitals is given below.
σ1s<σ⋆1s<σ2s<σ⋆2s<σpz<(π2px≈π2py)<(π⋆2px≈π⋆2py)<σ⋆2pz
Different atomic orbitalsof one atom combine with those atoms orbitals of the second atom which have comparable energies and proper orientation.
Further, if the overlapping is head on, the molecular orbital is called sigma, σ andif the overlap is lateral, the molecular orbital is called pi, π. The molecular orbitals are filled with electrons according to the same rules as followed for filling of atomic orbitals.
However, the order for filling is not the same for all molecules or their ions. Bond order is one of the most important parameters to compare the strength of bonds.
In which of the following molecules, σ2pz molecular orbital is filled after π2px and π2py molecular orbitals?
View SolutionMolecular orbitals are formed by the overlap of atomic orbitals. Two atomic called bonding molecular orbital (BMO) and anti - bonding molecular orbital (ABMO). Energy of anti - bonding molecular orbital (BMO) and anti - bonding molecular orbital ABMO). Energy of anti - bonding orbitals is raised above the parent atomic orbitals that have combined and the energy of the bonding orbital is lowered than the parent atomic orbitals. Energies of various molecular orbitals for elements hydrogen to nitrogen increase in the order : σ1s<σ∗1s<σ2s<σ∗2s<(π2px≈π2py)<σ2pz<(π∗p2px≈π∗2py)<σ∗2pz and for oxygen and fluorine order of energy of molecular orbitals is given as : σ1s<σ∗1s<σ2s<σ∗2s<σ2pz<(π2px≅2π2py)<(π∗2px≅π∗2py)<σ∗2pz. Different atomic orbitals of one atom combine with those atomic orbitals of the second atom which have comparable energies and proper orientation. Further, if the overlapping is head on, the molecular orbital is called 'sigma', (σ) and if the overlap is lateral, the molecular orbital is called 'pi', (π). The molecular orbitals are filled with electrons according to the same rules as followed for filling of atomic orbitals. However, the order for filling is not the same for all moleculas or their ions. Bond order is one of hte most important parameters to compare the strength of bonds.
In which of the following molecules, σ2pz molecular orbital is filled after π2px and π2py molecular orbitals?
View SolutionWhich of the molecule has p-p overlapping ?
View SolutionIn which of the following molecules, σ2pz molecular orbital is filled after π2pxandπ2py molecular orbitals ?
View Solution
