On the basic intermolecular force predict the correct order of decreasing bolling point of the compound ?
A
CH2OH>H2CH4
B
CH3OH>CH4H2
C
CH4CH2OH>H2
D
H2>CH4>CH2OH
Video Solution
Text Solution
The correct Answer is:b
Alcohol involves H bonding also molar of CH4>molofH2 greater is molar we of covalent compound hight is b.p
|
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:A2ZChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:Bond Enthalpy, Bond Angle And Bond Length
Explore 23 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:A2ZChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:Section B - Assertion Reasoning
Explore 37 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:A2ZChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:Molecular Orbitial Theory
Explore 42 Videos
- ATOMIC STRUCTUREBook:A2ZChapter:ATOMIC STRUCTUREExercise:Section D - Chapter End Test
Explore 30 Videos
- CHEMICAL EQUILIBRIUMBook:A2ZChapter:CHEMICAL EQUILIBRIUMExercise:Section D - Chapter End Test
Explore 30 Videos
Similar Questions
Knowledge Check
Correct order of decreasing boiling points is :
AHF>HI>HBr>HClBH2O>H2Te>H2Se>H2SCBr2>Cl2>F2DCH4>GeH4>SiH4Correct order of decreasing boiling points is :
AHF>HI>HBr>HClBH2O>H2Te>H2Se>H2SCBr2>Cl2>F2DCH4>GeH4>SiH4
The correct order of decreasing basicity of the above compounds is :AI>II>III>IVBII>I>IV>IIICIII>IV>II>IDII>I>III>IV
The correct order of increasing basicity for the following compounds is
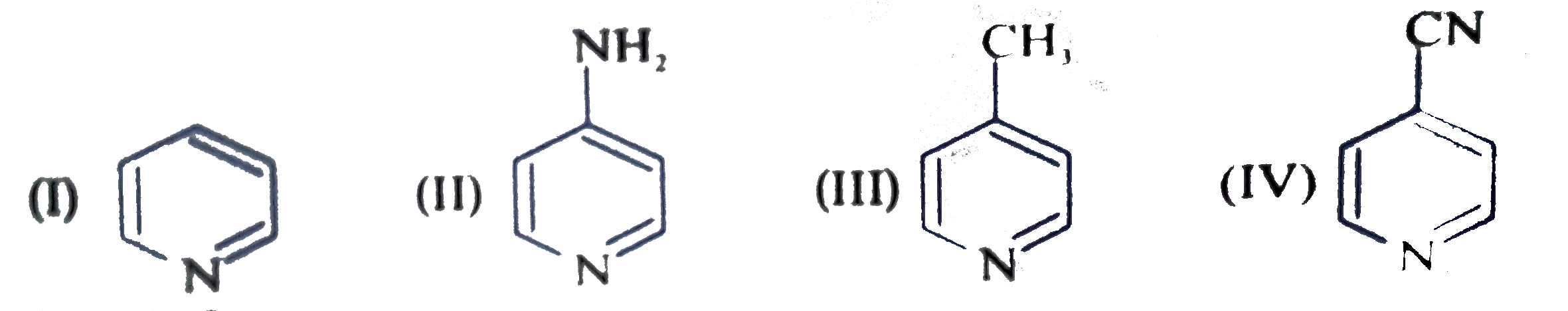
View Solution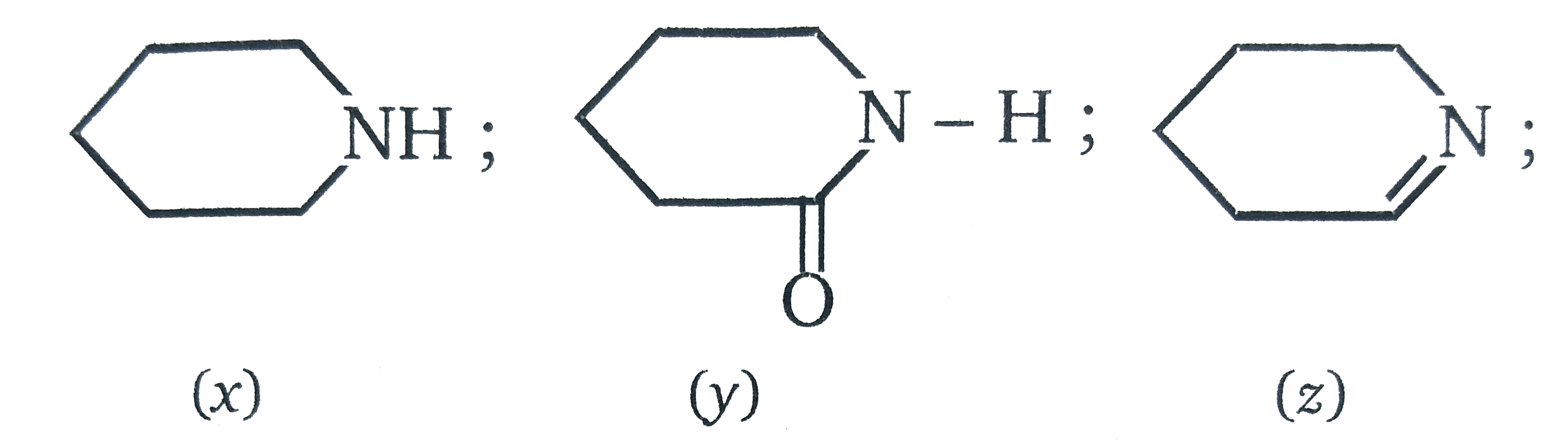
The correct order of decreasing basic strength of x,y, and z is :
View SolutionWhich is the correct decreasing order of basic strength in aqueous medium
View SolutionThe correct order of basicity of the following compounds is

View Solution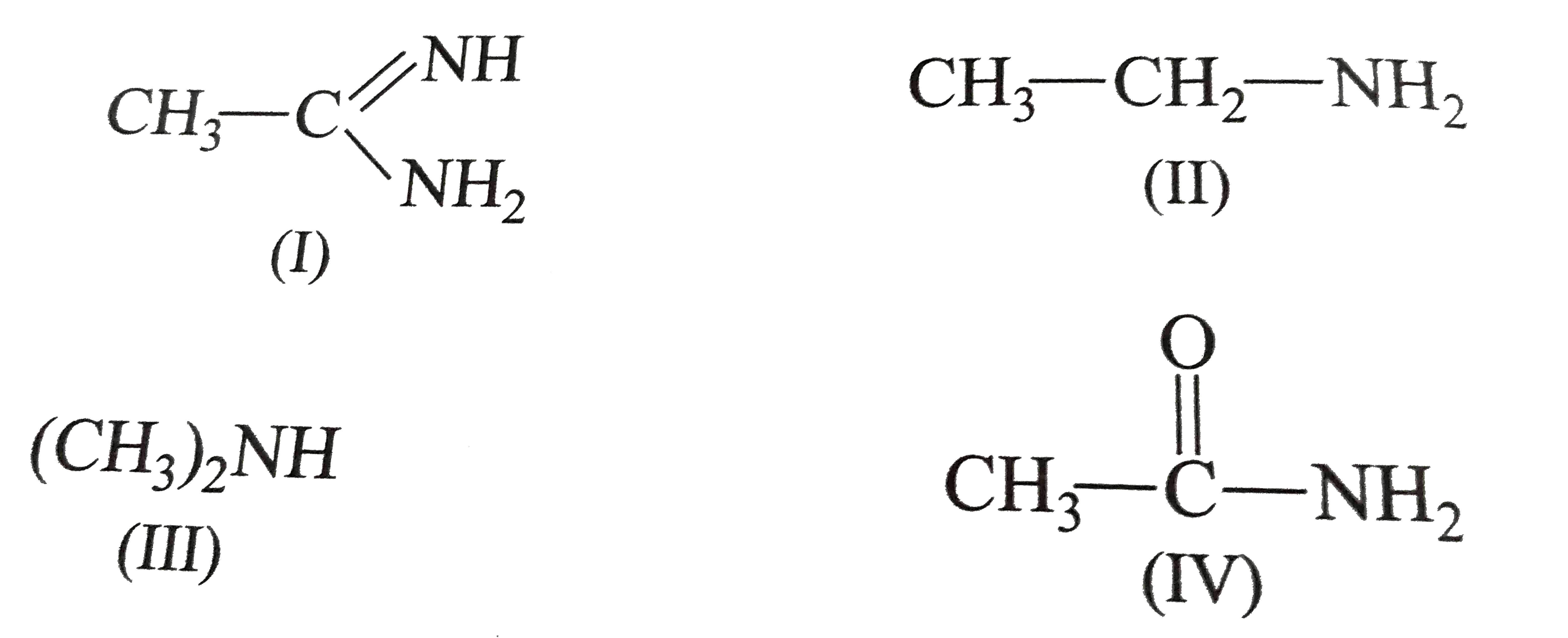
What is the correct order of basicity among the following compounds?
View Solution


