A reaction between methyl magnesium bromide and ethyl alcohol gives
Butane
Ethane
Propane
Methane
The correct Answer is:D
To solve the problem of the reaction between methyl magnesium bromide (a Grignard reagent) and ethyl alcohol, we can follow these steps:
1. Identify the Reactants:
- Methyl magnesium bromide (CH3MgBr)
- Ethyl alcohol (C2H5OH)
2. Understand the Nature of Grignard Reagents:
- Grignard reagents are highly reactive organomagnesium compounds that can react with various electrophiles, including alcohols.
3. Write the Reaction:
- When methyl magnesium bromide reacts with ethyl alcohol, the Grignard reagent acts as a nucleophile and attacks the electrophilic carbon of the hydroxyl group (–OH) in ethyl alcohol.
4. Mechanism of Reaction:
- The hydrogen atom from the hydroxyl group of ethyl alcohol is replaced by the methyl group (CH3) from the Grignard reagent.
- This results in the formation of methane (CH4) and the corresponding magnesium alkoxide (C2H5OMgBr).
5. Write the Products:
- The products of the reaction are:
- Methane (CH4)
- Magnesium bromide alkoxide (C2H5OMgBr)
6. Final Answer:
- The main product of the reaction is methane (CH4).
Summary of the Reaction:
The reaction between methyl magnesium bromide and ethyl alcohol produces methane (CH4) and magnesium bromide alkoxide.
- Methyl magnesium bromide (CH3MgBr)
- Ethyl alcohol (C2H5OH)
- Grignard reagents are highly reactive organomagnesium compounds that can react with various electrophiles, including alcohols.
- When methyl magnesium bromide reacts with ethyl alcohol, the Grignard reagent acts as a nucleophile and attacks the electrophilic carbon of the hydroxyl group (–OH) in ethyl alcohol.
- The hydrogen atom from the hydroxyl group of ethyl alcohol is replaced by the methyl group (CH3) from the Grignard reagent.
- This results in the formation of methane (CH4) and the corresponding magnesium alkoxide (C2H5OMgBr).
- The products of the reaction are:
- Methane (CH4)
- Magnesium bromide alkoxide (C2H5OMgBr)
- The main product of the reaction is methane (CH4).
The reaction between methyl magnesium bromide and ethyl alcohol produces methane (CH4) and magnesium bromide alkoxide.
Similar Questions
Knowledge Check
The reaction of methyl magnesium bromide with ethyl alcohol gives
Amethyl alcoholBmethaneCisopropyl alcoholDethanePhenyl magnesium bromide reacting with t-butyl alcohol gives
APh-OHBPh-HC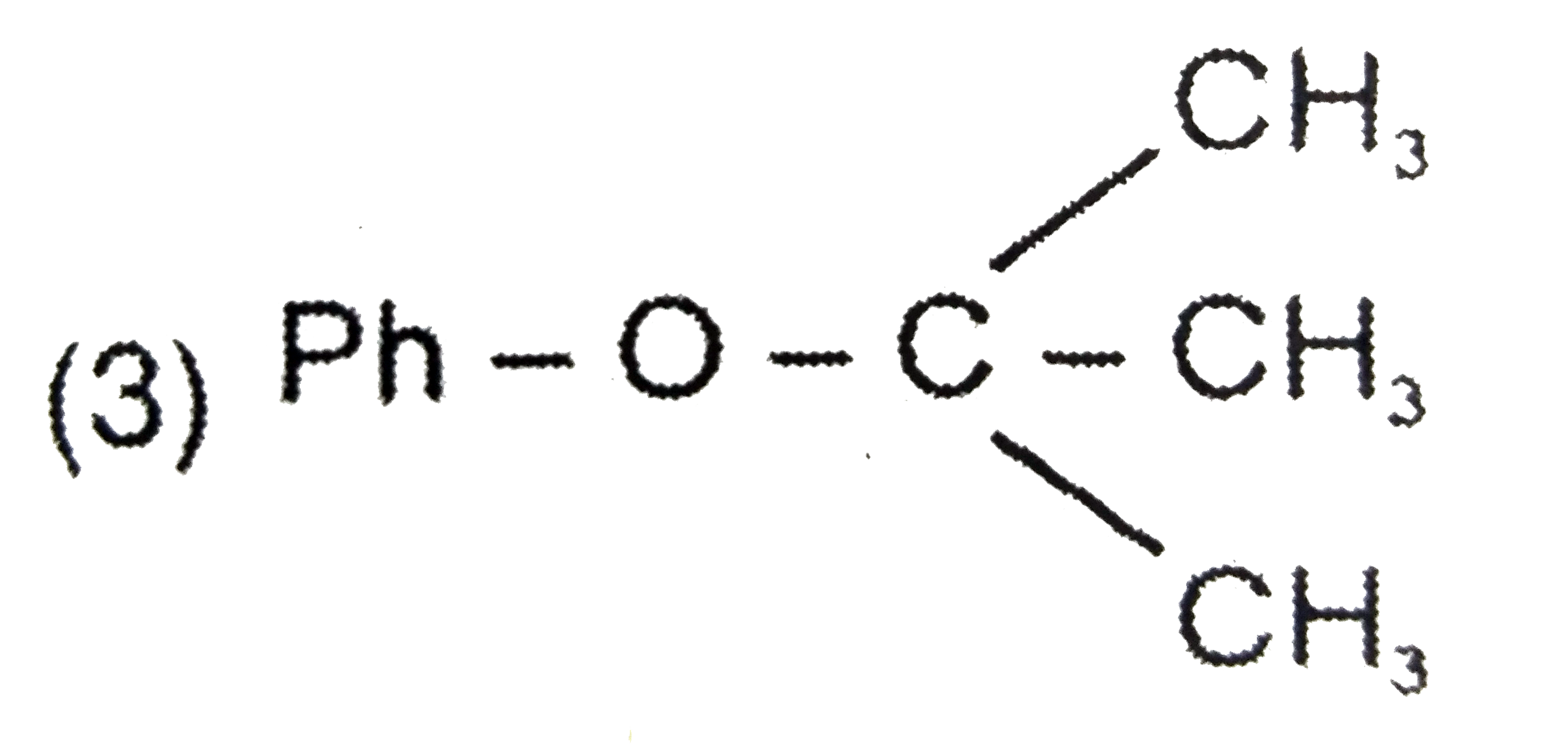 D
D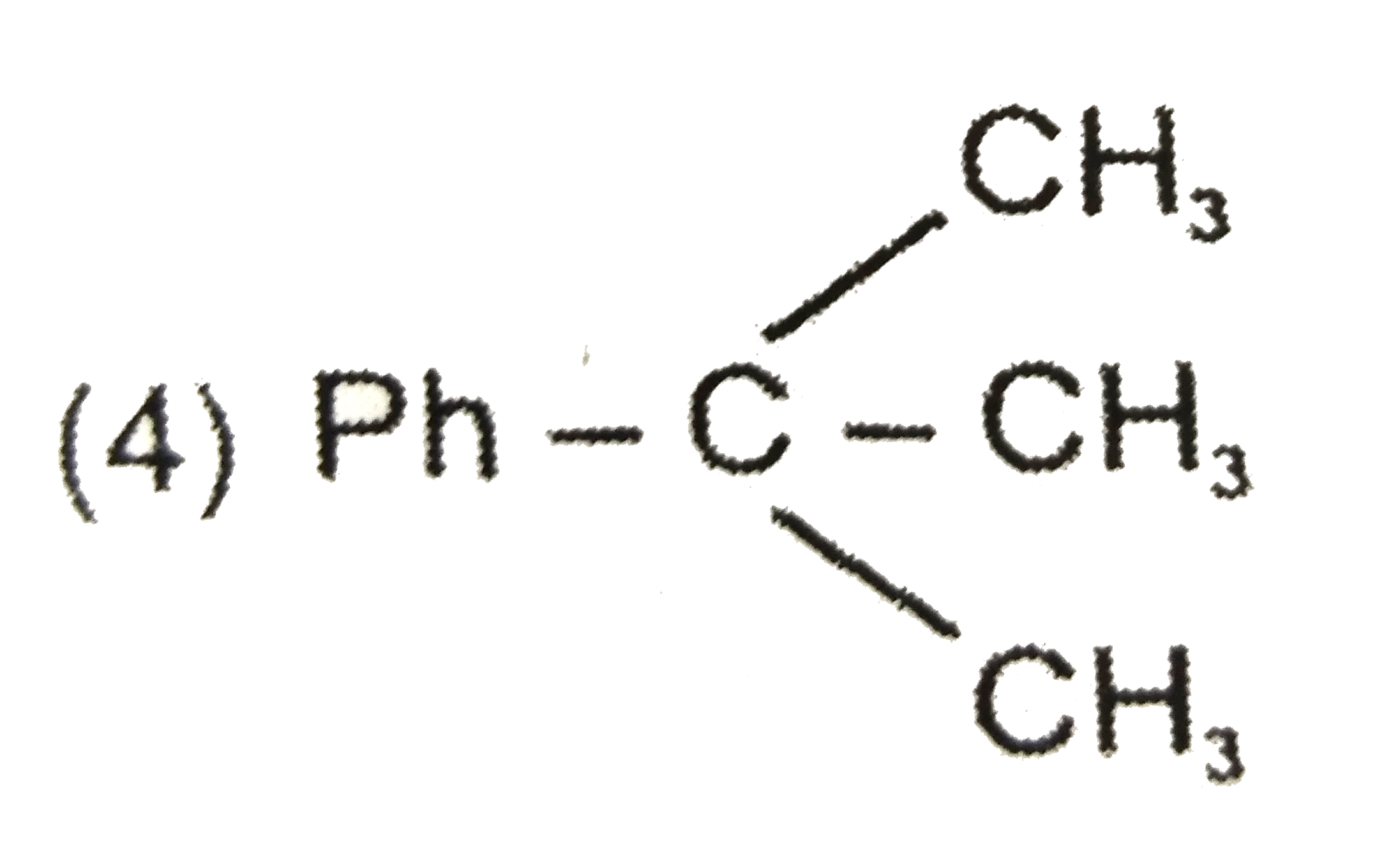
Reaction between phenyl magnesium bromide and methanol produces
AA mixture of anisole and Mg(OH)BrBA mixture of benzene and Mg(OME)BrCA mixture of toluene and Mg(OH)BrDA mixture of phenol and Mg(Me)Br
The compound obtained by the hydrolysis of the adduct formed by the reaction of ethyl magnesium bromide and acetadehyde is :
View SolutionReaction between dry ice and methyl magnesium bromide gives an addition compound which acidification yields:
View SolutionThe reactant (X) used in the following reaction is methyl magnesium bromide +X→2−methyl propan - 2 - ol
View SolutionEthyl magnesium bromide and chloramine react to produce
View SolutionWhich compound on reaction with ethyl magnesium bromide and water will form 2-methylbutan-2-ol ?
View Solution
