A mixture of 1− chloropropane and 2− chloropropane which treated with alcoholic KOH gives
A
1−propene
B
2−propene
C
Isoprepylene
D
All the three
Video Solution
Text Solution
The correct Answer is:a
CH3CH2CH2Cl+KOH→CH3−CH=CH2+KCl+H2O
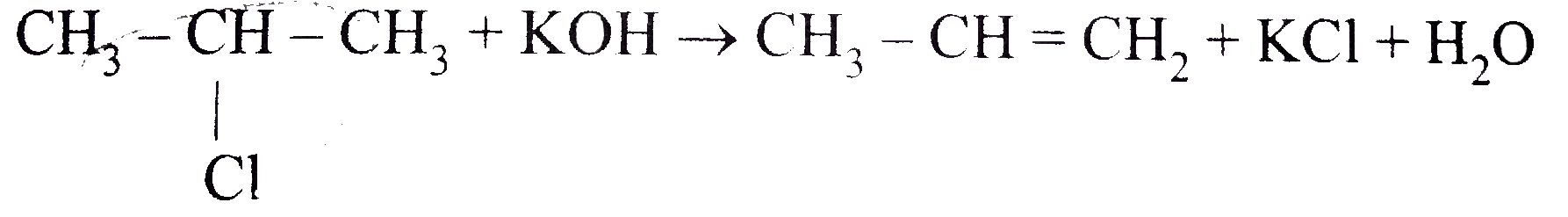
|
Similar Questions
Knowledge Check
A mixture of 1−chloropropane and 2−chloropropane when treated with alcoholic KOH, it gives
A1−PropeneB2−PropeneCIsopropyleneDA mixture of 1−propene and 2−propeneA mixture of 1−chloropropane and 2−chloropropane when treated with alcoholic KOH, it gives
A1-propeneB2-propeneCIsopropyleneDAll of the aboveA mixture of 1-chloropropane and 2-chloropropane when treated with alcoholic KOH gives
A1-propeneB2-propeneCIsopropyleneDAll of the above
A mixture of 1-chlorobutane and 2-chlorobutane when treated with alcoholic KOH gives:
View SolutionChloroform with alcoholic KOH gives
View SolutionPropyl bromide on reaction with alcoholic KOH gives-
View SolutionEthyl bromide on treatment with alcoholic KOH gives
View SolutionGem dihalides on treatment with alcoholic KOH give
View Solution

