Ph−ch2−ch=ch2dilH2SO4−−−−−→X,
Identify product 'X' is :
Ph−CH2−CH2−CH2−OH
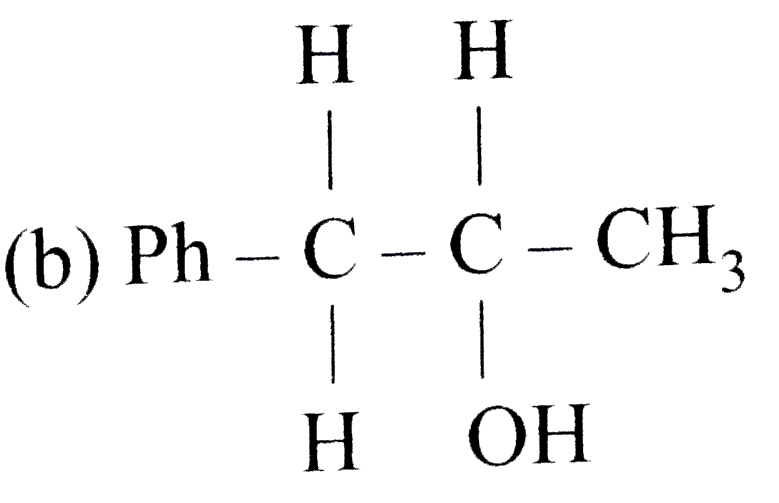
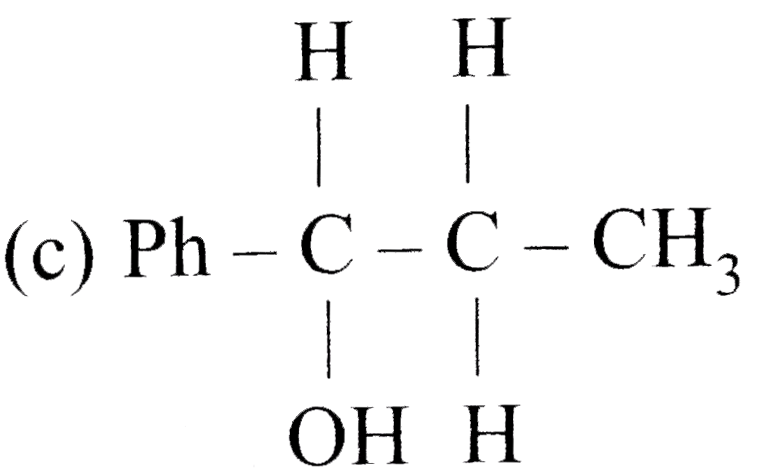
Ph−CH2−OH
The correct Answer is:c
To solve the question regarding the reaction of the compound Ph-CH2-CH=CH2 with dilute H2SO4, we will follow these steps:
Step 1: Identify the Compound
The compound given is Ph-CH2-CH=CH2, which consists of a phenyl group attached to a propene structure.
Step 2: Understand the Reaction Conditions
The reaction is carried out in the presence of dilute sulfuric acid (H2SO4). Dilute sulfuric acid acts as an acid catalyst and can protonate the alkene, leading to the formation of a carbocation.
Step 3: Protonation of the Alkene
In the presence of dilute H2SO4, the double bond in the alkene will undergo protonation. The double bond between CH and CH2 will break, and a proton (H+) from the acid will add to one of the carbon atoms of the double bond, forming a carbocation.
Step 4: Formation of Carbocation
The protonation leads to the formation of a more stable carbocation. The structure after protonation will be Ph-CH2-C+(H)-CH3 (where C+ is the positively charged carbon).
Step 5: Nucleophilic Attack by Water
Next, the hydroxide ion (OH−) from the dissociation of sulfuric acid will act as a nucleophile and attack the carbocation. This will lead to the formation of an alcohol.
Step 6: Final Product Formation
The final product after the nucleophilic attack will be Ph-CH2-CH(OH)-CH3. This compound is an alcohol, specifically phenylpropan-2-ol.
Conclusion
Thus, the product X formed from the reaction of Ph-CH2-CH=CH2 with dilute H2SO4 is phenylpropan-2-ol.
Final Answer
The product X is Ph-CH2-CH(OH)-CH3.
---
The compound given is Ph-CH2-CH=CH2, which consists of a phenyl group attached to a propene structure.
The reaction is carried out in the presence of dilute sulfuric acid (H2SO4). Dilute sulfuric acid acts as an acid catalyst and can protonate the alkene, leading to the formation of a carbocation.
In the presence of dilute H2SO4, the double bond in the alkene will undergo protonation. The double bond between CH and CH2 will break, and a proton (H+) from the acid will add to one of the carbon atoms of the double bond, forming a carbocation.
The protonation leads to the formation of a more stable carbocation. The structure after protonation will be Ph-CH2-C+(H)-CH3 (where C+ is the positively charged carbon).
Next, the hydroxide ion (OH−) from the dissociation of sulfuric acid will act as a nucleophile and attack the carbocation. This will lead to the formation of an alcohol.
The final product after the nucleophilic attack will be Ph-CH2-CH(OH)-CH3. This compound is an alcohol, specifically phenylpropan-2-ol.
Thus, the product X formed from the reaction of Ph-CH2-CH=CH2 with dilute H2SO4 is phenylpropan-2-ol.
The product X is Ph-CH2-CH(OH)-CH3.
Similar Questions
Knowledge Check
Ph−CH2−CH=CH2dil.H2SO4−−−−−−→A,A is
APh−CH2−CH2−CH2−OHBPh−CH2−CH∣OH−CH3CPh−CH∣OH−CH2−CH3DPh−CH2−OHPh−CH2−CH=CH2तनु H2SO4−−−−−−→A,A है:
APh−CH2−CH2−CH2−OHBPh−CH2−C∣OHH−CH3CPh−C∣OHH−CH2−CH3DPh−CH2−OHConsider the following sequence of reactions
CH2=CH2PhCO3H−−−−−−→CH2Cl2Adil.H2SO4−−−−−−→B
The product (B) isAPhCH2CH2OHBCH2OHCH2OHC(CH3)2CHOCH2CH3DCH2OHCH2Cl
CH3COCH2COOC2H5NaBH4−−−−→X
The product X is
View SolutionPh−CH=CH=PhCI2−−→CI4X2NaNH2−−−−→YH2,Pd−CaCO3−−−−−−−−−→Z
Identify product (Z) of the reaction.
View SolutionIn the reaction
Ph−C≡C−CH3H3O+−−−→HgSO4A, A is
View SolutionCH3−CH∣OH−CH2−CH3conc.H2SO4−−−−−−−→Product(I)+Product(II)
What is not true regarding the products?
View SolutionCH3−CH2−⋅⋅O⋅⋅−CH=CH2H2∅H2SO4−−−−−→
The∏ucts/sformed∈thereactionis/are:
View Solution
