(CH3)2C=CHCH3+NOBr→ Product . The structure of the product is
A
(CH3)2C(NO)−CH(Br)CH3
B
(CH3)2C(Br)−CH(NO)CH3
C
(CH3)2CH−C(NO)(Br)CH3
D
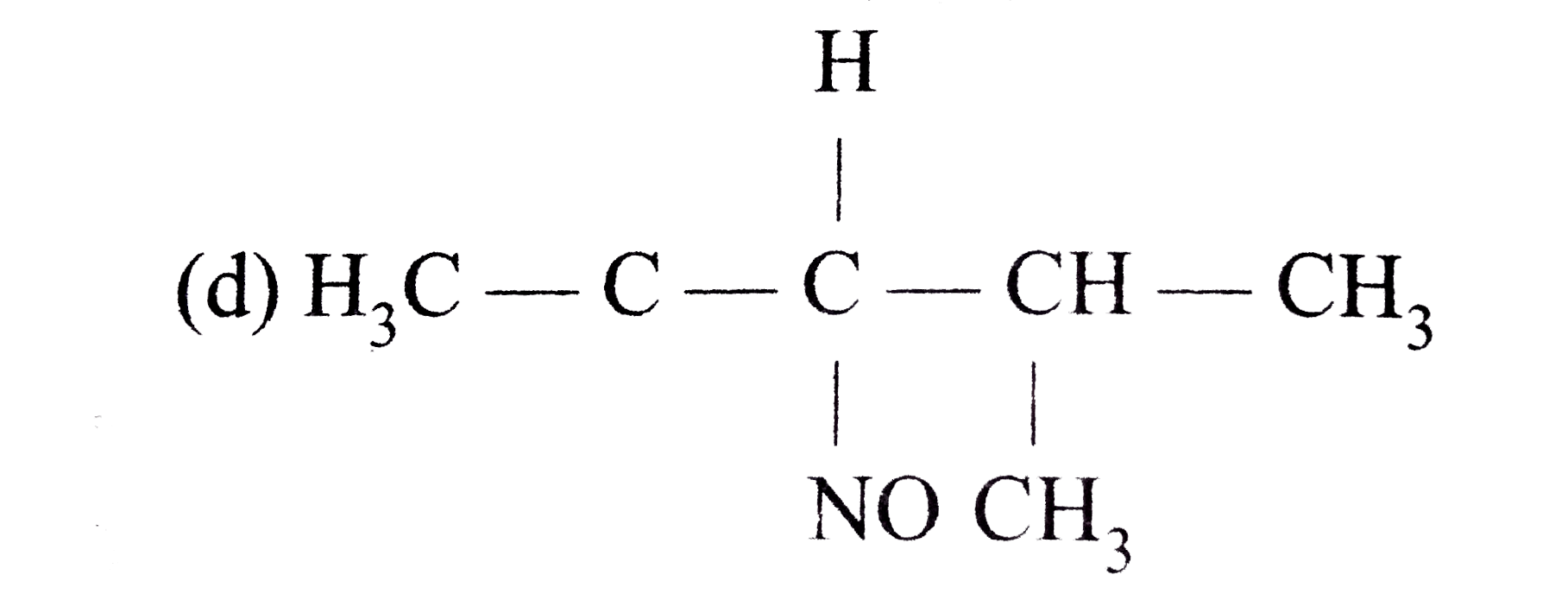
Video Solution
Text Solution
The correct Answer is:b
The reaction follows Markownikoff rule, namely the bromide ion adds on to the carbon having the least number of hydrogen and the more positive part namely the −NO group adds to the other carbon of the double bond.
|
Similar Questions
Knowledge Check
_S01_838_Q01.png) +CH3NH2→ Product: product is :A
+CH3NH2→ Product: product is :A_S01_838_O01.png) B
B_S01_838_O02.png) C
C_S01_838_O03.png) DNone of these
DNone of theseCH2=CH−CH=CH2CI3Br−−−→ product.The major product is:
ABr−CH2CH=CH−CH2−CI3BCH2=CH−C∣BrH−CH2−CI3CCH3−C∣ODH−CH3Dnone is correctIn the reaction
(CH3)2C−CH=CH2+HBr→
the chief product isA(CH3)2C∣Br−CH(CH3)2B(CH3)3C−CH∣Br−CH3C(CH3)3CH2CH2BrD(CH3)2CHC∣CH3HBr
In the reaction
CH3C≡C−Na++(CH3)2CHCl → Product the product fomed is :
View SolutionIn the given
 +CH5−CH2−CH2−ClAlCl3−−→ Product the major product will be
+CH5−CH2−CH2−ClAlCl3−−→ Product the major product will be
View SolutionCH3–NH2+CHCl3+KOH→ major product will be :
View SolutionFor the reaction C6H6+(CH3)2CHCH2ClAnhy.AlCl3−−−−−−→
The final product is
View Solution(CH3)3CCH2−CBr(CH3)2Heat−−→
The major product is
View Solution
