Which of the following alkene in acid catalysed hydration form 2−methyl propan −2−ol ?
(CH3)2CH=CH2
CH3CH=CH2
CH3−CH=CH−CH3
CH3−CH2−CH=CH2
The correct Answer is:A
To determine which alkene will form 2-methylpropan-2-ol upon acid-catalyzed hydration, we will analyze each option provided.
1. Identify the Alkenes: We have four alkenes to consider:
- A: CH3-CH=CH2
- B: CH3-CH=CH-CH2
- C: CH3-CH=CH-CH3
- D: CH3-CH2-CH=CH2
2. Apply Acid-Catalyzed Hydration: In acid-catalyzed hydration, the alkene reacts with water in the presence of an acid catalyst. According to Markovnikov's rule, the hydroxyl group (OH) will attach to the carbon with the greater number of hydrogen atoms, while the hydrogen (H) will attach to the carbon with fewer hydrogen atoms.
3. Analyze Each Alkene:
- Option A (CH3-CH=CH2):
- The double bond between the second and third carbon will break.
- OH will attach to the second carbon (more substituted), and H will attach to the third carbon.
- Product: CH3-CHOH-CH3 (which is 2-propanol, not 2-methylpropan-2-ol).
- Option B (CH3-CH=CH-CH2):
- The double bond between the second and third carbon will break.
- OH will attach to the second carbon, and H will attach to the third carbon.
- Product: CH3-CHOH-CH2-CH3 (which is not 2-methylpropan-2-ol).
- Option C (CH3-CH=CH-CH3):
- The double bond between the second and third carbon will break.
- OH can attach to either the second or third carbon since it is symmetrical.
- Product: CH3-CHOH-CH2-CH3 (which is not 2-methylpropan-2-ol).
- Option D (CH3-CH2-CH=CH2):
- The double bond between the third and fourth carbon will break.
- OH will attach to the third carbon (more substituted), and H will attach to the fourth carbon.
- Product: CH3-CH2-CHOH-CH3 (which is 2-methylpropan-2-ol).
4. Conclusion: After analyzing all options, we find that Option D is the correct answer as it forms 2-methylpropan-2-ol.
Final Answer:
The alkene that forms 2-methylpropan-2-ol upon acid-catalyzed hydration is Option D: CH3-CH2-CH=CH2.
- A: CH3-CH=CH2
- B: CH3-CH=CH-CH2
- C: CH3-CH=CH-CH3
- D: CH3-CH2-CH=CH2
- Option A (CH3-CH=CH2):
- The double bond between the second and third carbon will break.
- OH will attach to the second carbon (more substituted), and H will attach to the third carbon.
- Product: CH3-CHOH-CH3 (which is 2-propanol, not 2-methylpropan-2-ol).
- The double bond between the second and third carbon will break.
- OH will attach to the second carbon, and H will attach to the third carbon.
- Product: CH3-CHOH-CH2-CH3 (which is not 2-methylpropan-2-ol).
- The double bond between the second and third carbon will break.
- OH can attach to either the second or third carbon since it is symmetrical.
- Product: CH3-CHOH-CH2-CH3 (which is not 2-methylpropan-2-ol).
- The double bond between the third and fourth carbon will break.
- OH will attach to the third carbon (more substituted), and H will attach to the fourth carbon.
- Product: CH3-CH2-CHOH-CH3 (which is 2-methylpropan-2-ol).
The alkene that forms 2-methylpropan-2-ol upon acid-catalyzed hydration is Option D: CH3-CH2-CH=CH2.
Similar Questions
Knowledge Check
Which of the following alkene on acid catalysed hydration form propan-2-ol
ACH3CH=CH2B(CH3)2C=CH2CCH3CH=CHCH3DCH3CH2CH=CH2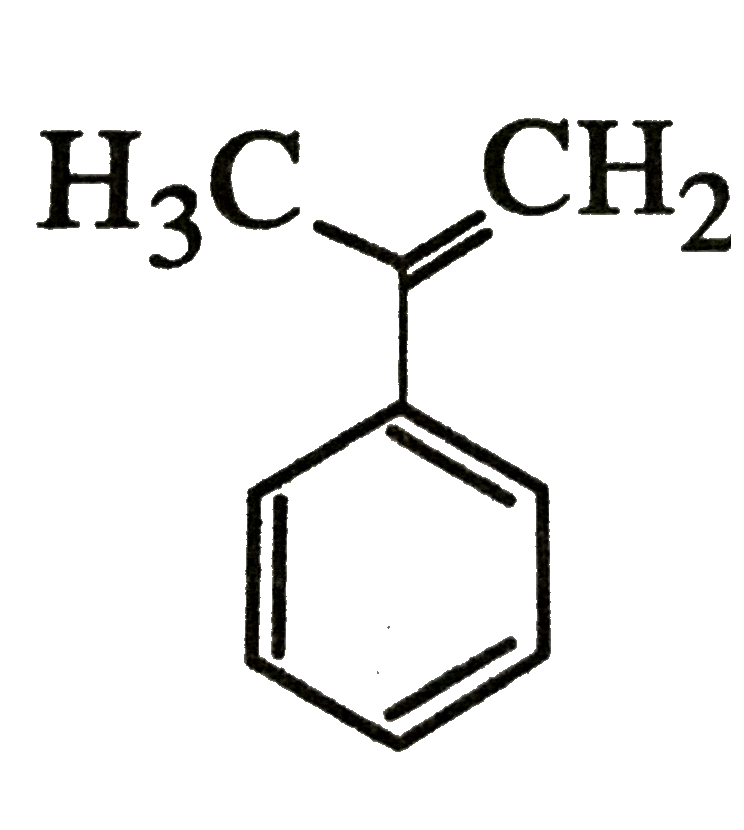 on acid catalysed hydration givesA
on acid catalysed hydration givesA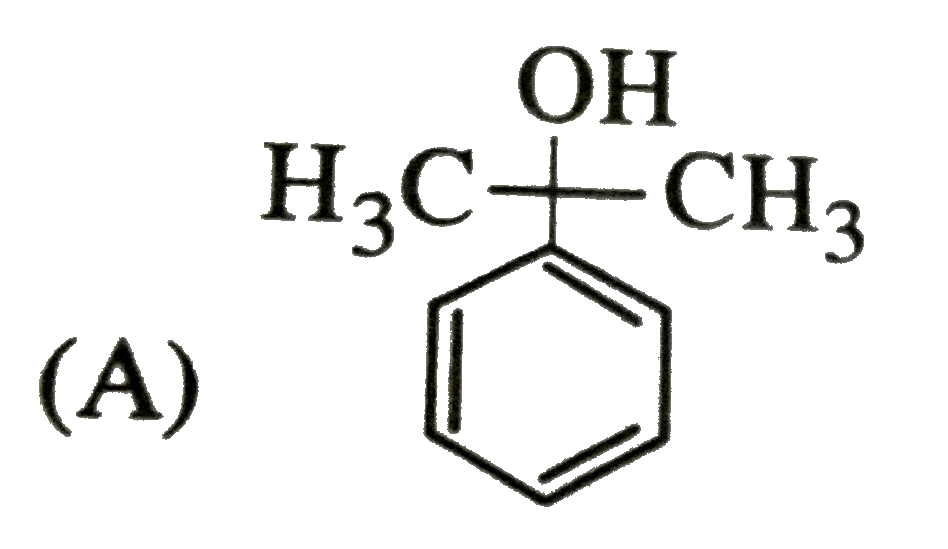 B
B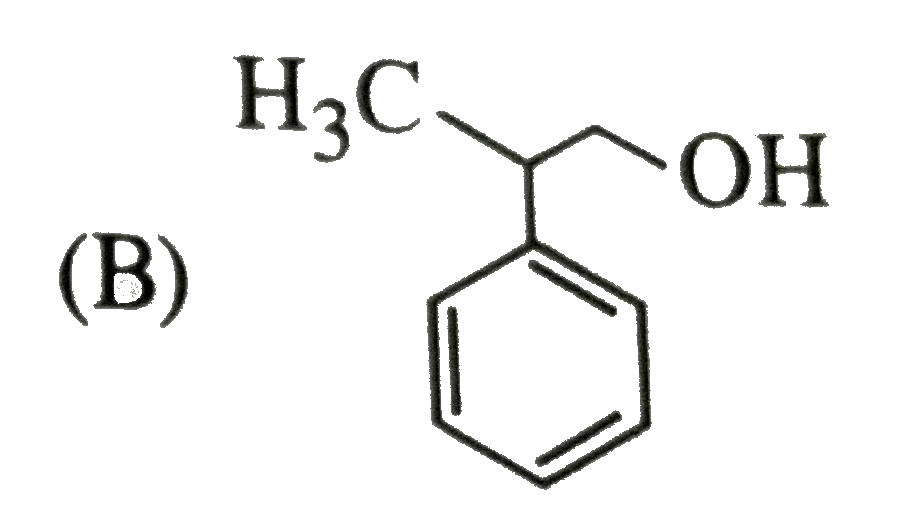 C
C DNone of these
DNone of theseWhich of the following alkenes on acid-catalysed hydration gives a tertiary alcohol ?
ABut -2-eneBPropeneCBut-1-eneD2-Methylpropene
The product of acid catalysed hydration of 2-phenylpropene is
View SolutionThe product of acid catalyzed hydration of 2−pheny1propene is
View SolutionThe major product obtained on acid-catalysed hydration of 2-phenylpropane is
View SolutionWhich of the following reactions with yield propan -2-ol ?
View SolutionWhich of the following is not the characterstic of acid catalyzed hydration of alkenes?
View Solution

