The relationship between PC,VC and TC is
PCVC=RT
PCVC=3RTC
PCVC=35RTC
PCVC=38RTC
The correct Answer is:D
Step by step video, text & image solution for The relationship between P_(C), V_(C) and T_(C) is by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The correct relationship between TC,TB and Ti is -
ATC<TB<TiBTC>TB>TiCTC<Ti<TBDTi<TB<TCThe relationship between KpandKc is correctly shown as
AKc=Kp(RT)ΔnBKp=Kc(RT)−ΔnCKp=Kc(RT)ΔnDKc=Kp(RT)−ΔnGiven that for a reaction of nth order, the integrated rate equation is:
K=1t(n−1)[1Cn−1−1Cn−10], where C and C0 are the concentration of reactant at time t and initially respectively. The t3/4 and t1/2 are related as t3/4 is time required for C to become C1/4) :At3/4=t1/2[2n−1+1]Bt3/4=t1/2[2n−1−1]Ct3/4=t1/2[2n−1−1]Dt3/4=t1/2[2n+1+1]
In the P - V diagram, the point B and C correspond to temperatures T1andT2 respectively. State relationship between T1andT2.
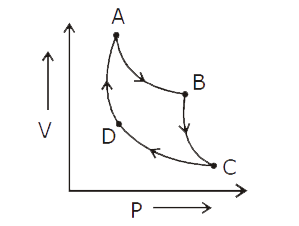
View SolutionFind the critical constant (Pc,Vc and Tc) in terms of A and B, also find compressibility factor ( z) for the following equation of state.
PV=RT−AV+2BV2
where && and B are constant, P =pressure and V=molar volume.
View SolutionWhich one among the following is the correct option for right relationship between Cp and Cv for one mole of ideal gas?
View SolutionThe relationship between equilibrium constants KpandKc for a gaseous reaction is:
View SolutionThe difference between ¯¯¯Cpand¯¯¯Cv is [¯¯¯Cpand¯¯¯Cv signify molar quantities ]
View Solution

