In an isothermal reversible cyclic process, the total change in internal energy
is always positive
is always negative
can be positive or negative
is always zero
The correct Answer is:D
For cyclic process, ΔV=0,ΔU=0,ΔH=0.
Similar Questions
Knowledge Check
In a reversible isothermal process, the change in internal energy is :
AzeroBpositiveCNegativeDNone of the aboveIn a cyclic process the change in the internal energy of system is
Amaximum but not zeroBmaximum but not infiniteCzeroDinfinite specific heatStatement: In the case of an ideal gas the changes in Gibbs and Helmholtz free energies are equal to each other (ΔG=ΔA) for isothermal reversible process.
Explanation: There is no change in internal energies and enthalpies for ideal gas at constant temperature.AS is correct but E is wrong.BS is wrong but E are correct and ECBoth S and E are correct but E is correct explanation of S.DBoth S and E are correct but E is not correct explanation of S.
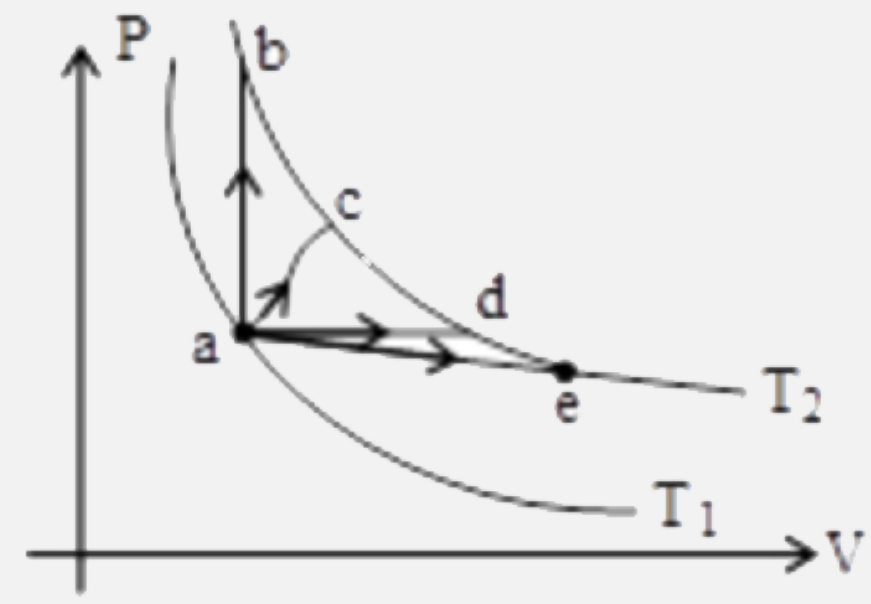
The figure shows two isotherms at temperatures T1 and T2. A gas is taken from one isotherm to another isotherm through different processes. Then change in internal energy ΔU has a relation -
View SolutionAssertion : In an isothemal expansion the gas absorbs heat and does work .
Reason: In an isothermal process there is no change in internal energy of an ideal gas.
View SolutionIn a cyclic process, the internal energy of the gas
View SolutionIn a cyclic process, the internal energy of the gas
View SolutionAssertion. Heat energy is completely transformed into work during the isothermal expansion of an ideal gas.
Reason . During an isothermal process, the change in internal energy of a gas due to decrease in pressure is nullified by the change due to increase in volume.
View Solution

