Relation between CpandCy (in calories) is
CP+CV=2
CV−CP=2
CP−CV=2
CPCV=2
The correct Answer is:C
Cp−Cv=RandR=2cal.
Similar Questions
Knowledge Check
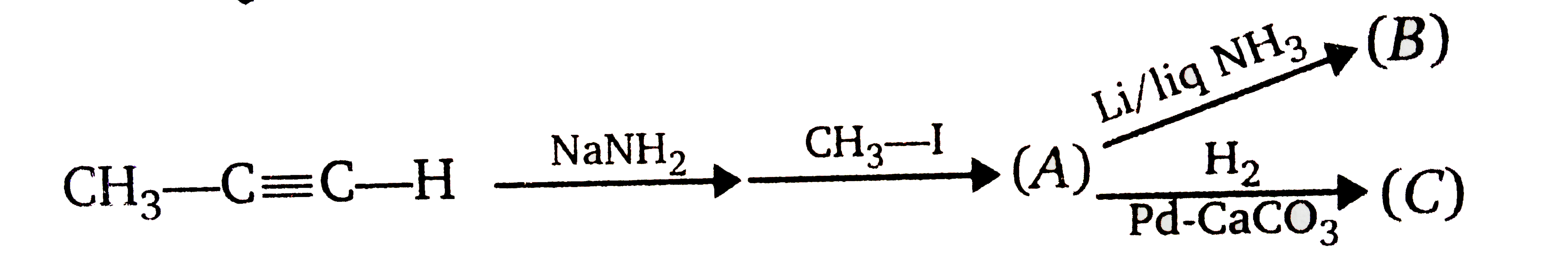
Relation between (B) and (C) is:AEnantiomerBDiastereomersCGeometrical isomerDMesoFor 2 mole of an ideal gas , the relation between Cp&Cv (non-molar) are:
ACp−Cv=2RBCv−Cp=2RCCp−Cv=RDCv−Cp=R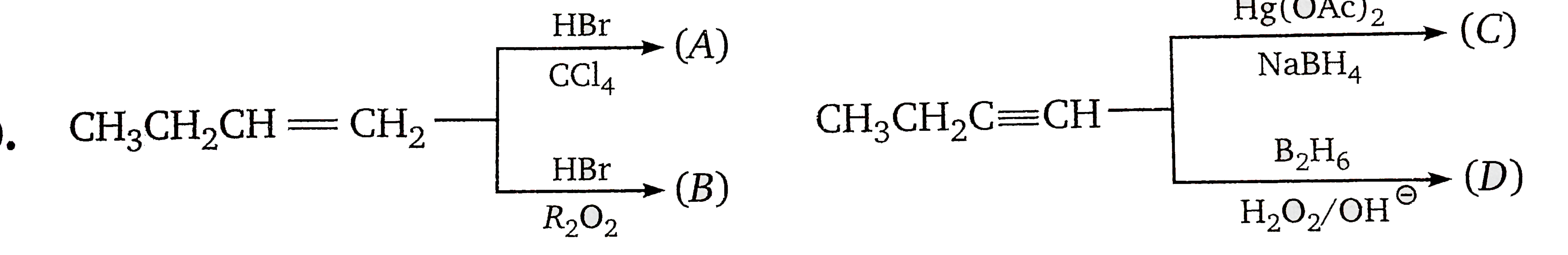
Relation between A and B, C and D are:APosition, chainBPosition, FunctionalCChain, IdenticalDMetamer, Functional
Heat capacity (Cp) is an extensive property but specific heat (C) is an intensive property.What will be the relation between Cp and C for 1 mol of water? (Keep your answer in J/K and in two digits only)
View SolutionHeat capacity (Cp) is an extensive property but specific heat (C) is an intensive property.What will be the relation between Cp and C for 1 mol of water? (Keep your answer in J/K and in two digits only)
View SolutionThe difference between Cp and Cv can be derived using the empirical relation H = U + pV. Calculate the difference between Cp and Cv for 10 moles of an ideal gas.
View SolutionHeat capacity (Cp) is an extensive property but specific heat (C) is an intensive property. What will be the relation between CpandC for 1 mole of water?
View SolutionThe relation between KPandKC for the reaction A(g)+B(g)⇔C(g)+2D(g) is -
View Solution
