Which product distills over when a mixture of absolute alcohol and glacial acetic acid is heated in presence of conc. H2SO4 or dry hydrogen chloride gas ?
A
Acetoacetic ester
B
Ethyl acetate
C
Ethyl acetoacetate
D
Methyl acetoacetate
Video Solution
Text Solution
The correct Answer is:B
CH3−O∣∣C−OH+HOC2H5conc.H2SO4−−−−−−−−−→dry HCl gasCH3−O∣∣C−OC2H5+H2O
|
Topper's Solved these Questions
- ALDEHYDES,KETONES AND CARBOXYLIC ACIDSBook:MARVEL PUBLICATIONChapter:ALDEHYDES,KETONES AND CARBOXYLIC ACIDSExercise:CARBOXYLIC ACIDS
Explore 198 Videos
- ALCOHOL,PHENOLS AND ETHERS Book:MARVEL PUBLICATIONChapter:ALCOHOL,PHENOLS AND ETHERS Exercise:Test your Grasp : Ethers
Explore 30 Videos
- BIOMOLECULESBook:MARVEL PUBLICATIONChapter:BIOMOLECULESExercise:TEST YOUR GRASP
Explore 40 Videos
Similar Questions
Knowledge Check
In acidic medium in presence of conc. H2SO4 acetaldehyde will give :
ACH3−OH∣CH−CH2CHOBCH3CH=CH−CHOC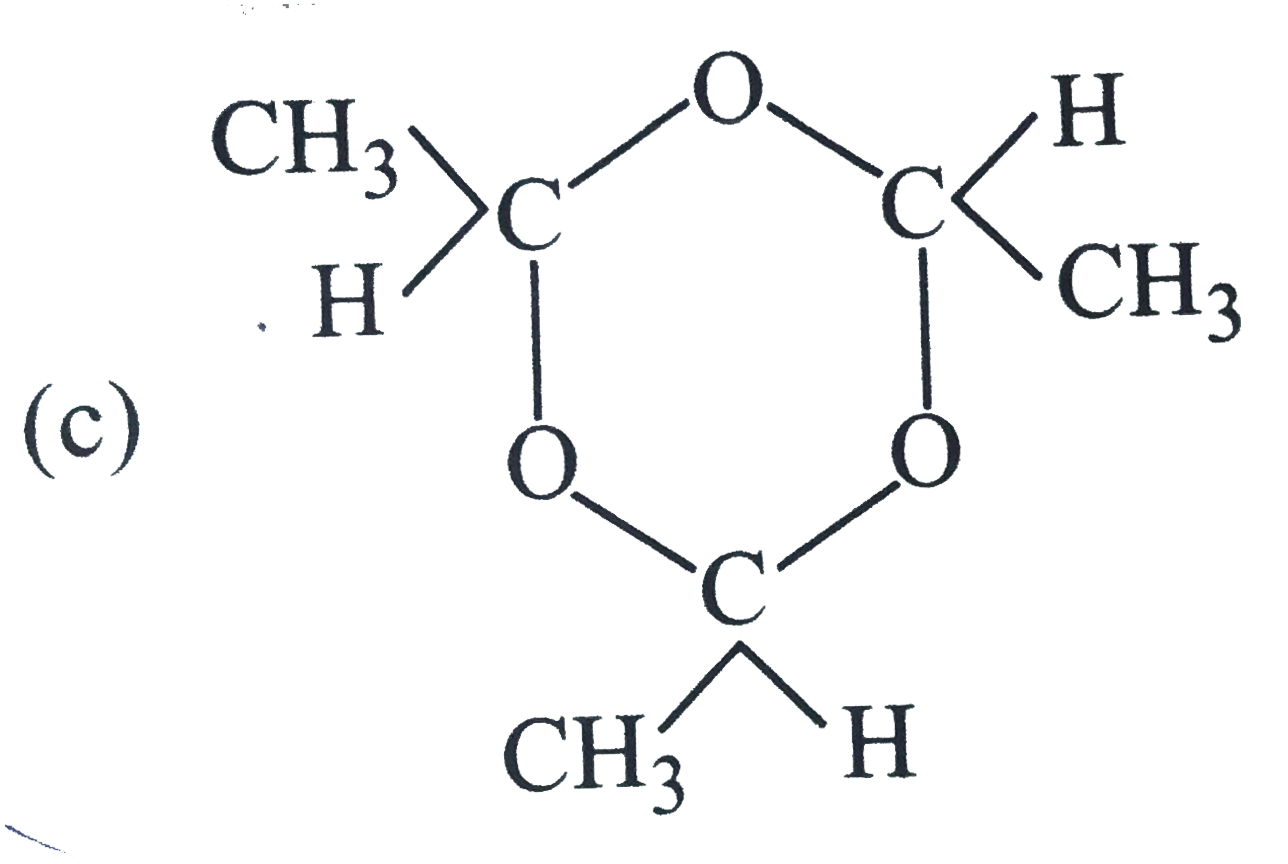 D CH3 H | |H−C−O−C−CH3 | | O O | |CH3−C−O−C−H | | H CH3
D CH3 H | |H−C−O−C−CH3 | | O O | |CH3−C−O−C−H | | H CH3Lactic acid on heating with conc. H2SO4 gives
AAcetic acidBPropionic acidCAcrylic acidDFormic acidLactic acid on heating with conc. H2SO4 gives
AAcetic acidBPropionic acidCAcrylic acidDFormic acid
Heating mxiture of ethyl alcohol and acetic acid in presence of conc. H2SO4 produces a fruity smelling compound. This reaction is called :
View SolutionAcetic acid on warming with hydrazoic acid in presence of conc. H2SO4 gives
View SolutionAcetic acid on warming with hydrazoic acid in presence of conc. H2SO4 gives :
View SolutionHeating mxiture of ethyl alcohol and acetic acid in presence of conc. H2SO4 produces a fruity smelling compound. This reaction is called :
View SolutionHeating mxiture of ethyl alcohol and acetic acid in presence of conc. H2SO4 produces a fruity smelling compound. This reaction is called :
View Solution
