Acetonitrile Hydrolysis−−−−−→P, then the compound P is
A
acetic acid
B
propanic acid
C
formic acid
D
butyric acid
Video Solution
Text Solution
The correct Answer is:A
CH3−C≡NHydrolysis−−−−−−−−−→H−OH/H+CH3−O∣∣C−OH Acetic acid
|
Similar Questions
Knowledge Check
CH3OHPI3−−→(A)KCN−−→(B)Hydrolysis−−−−−→(C).
The compound (C) is :ACH3OHBHCOOHCCH3CHODCH3COOHIn the following reaction, Q will be
Aceto nitrile Hydrolysis−−−−−−−−→(H+)PΔ,P2O5−−−−−−−−−−→(Dehydration)Q+H2OAacetica acidBacetic anhydrideCacetamideDacetaldehydeCH3OHPI3−−→(A)KCN−−→(B)Hydrolysis−−−−−→(C)
The compound (C) is:ACH3OHBHCOOHCCH3CHODCH3COOH
A OBr−−−−→CHBr3 the compound is :
View SolutionCH3OH+PCl3→A
AKCN−−→Bhydrolysis−−−−−→C. Then ''C'' is
View SolutionCH3CCl2CH3NaOH−−−−−−→HydrolysisP−H2O−−−→QOxidation−−−−−−−→[O]R
The compound R is
View Solution
Br2/heat−−−−−→ P, P is
View SolutionSOCl2−−−→(A)KCN−−→(B)H+−−→(C ), the compound (C ) is
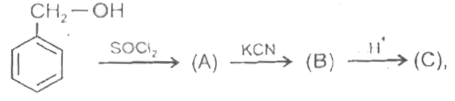
View Solution
