P - V plots for two gases, undergoing adiabatic processes are as shown in the figure.
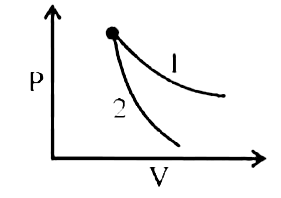
Plots 1 and 2 should correspond respectively to
A
He and O2
B
He and Ar
C
O2 and He
D
O2andN2
Video Solution
Text Solution
The correct Answer is:C
For an adiabatic process PVγ = constant
∴P⋅rVγ−1dV+VγdP=0
∴γPdV=−VdPordPdV=−γPV
∴ Slope of the graph ∝γ
In the given graph (slope)2>(slope)1orγ2>γ1
∴ 2 corresponds to monoatomic Helium [γ=1.67]
and 1 corresponds to diatomic O2 [γ=1.4]
|
Similar Questions
Knowledge Check
P-V plots for two gases during adiabatic processes are shown in the figure. Plots 1 and 2 should corresponds respectively to
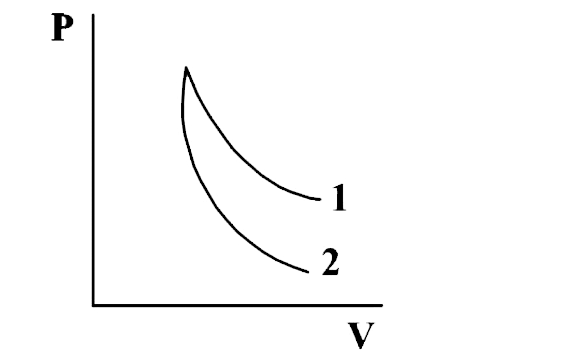 AHe and O2BO2 and HeCHe and ArDO2 and N2
AHe and O2BO2 and HeCHe and ArDO2 and N2P-V plots for two gases during adiabatic processes are shown in the figure. Plots 1 and 2 should corresponds respectively to
 AHeandO2BO2andHeCHeandArDO2andN2
AHeandO2BO2andHeCHeandArDO2andN2P−V plots for two gases during P4 adiabatic process are shown in the figure. Plots 1 and 2 should correspond respectively to
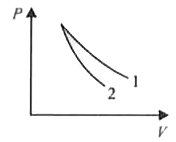 AHe and O2BO2 and HeCHe and ArDO2 and N2.
AHe and O2BO2 and HeCHe and ArDO2 and N2.
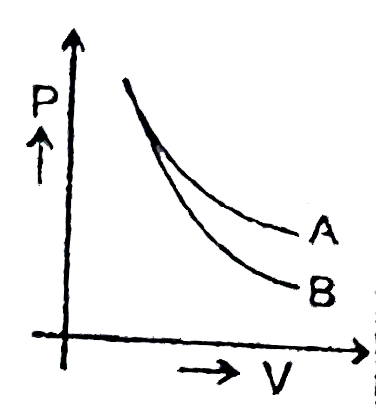
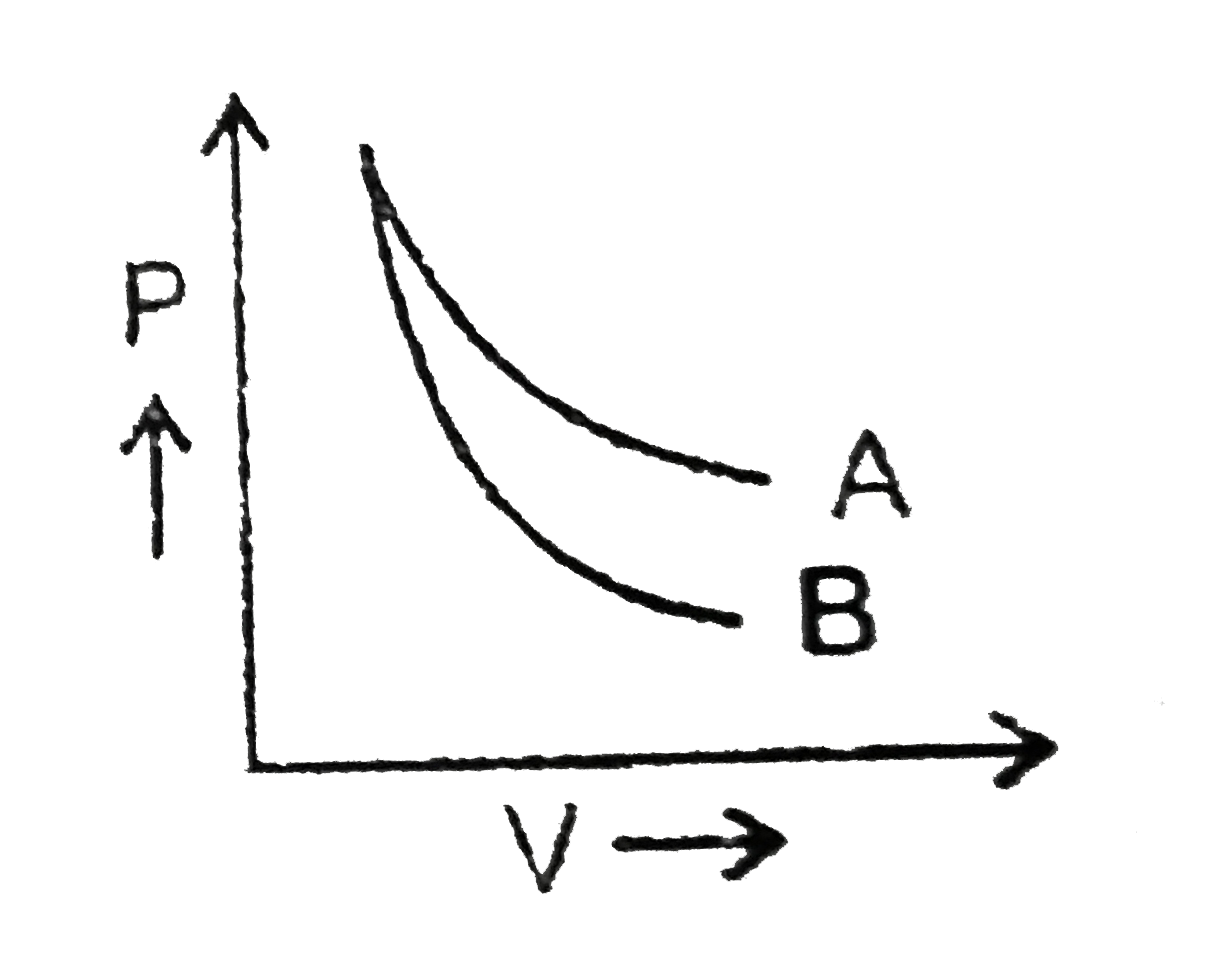
P-V plot for two gases (assuming ideal) during adiabatic processes are given in the figure. Plot A and plot B should correspond respectively to :
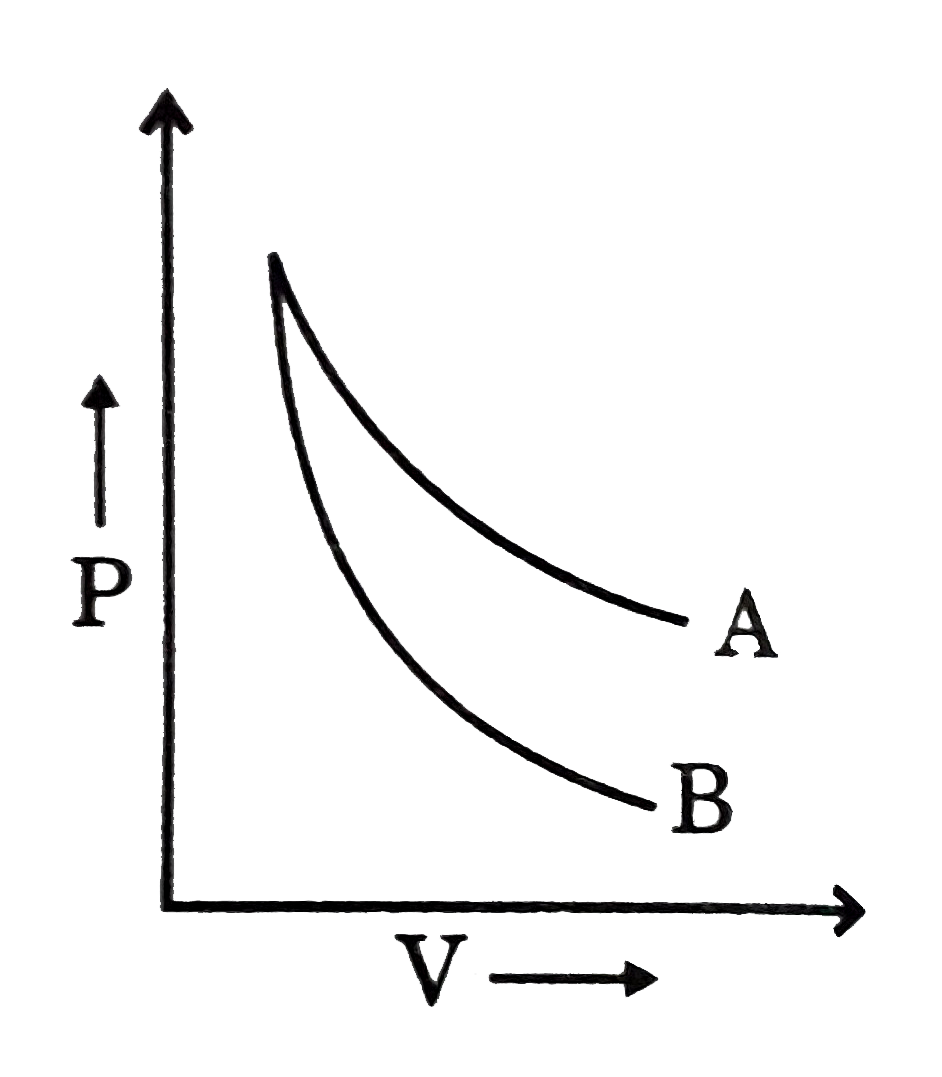
View SolutionP−V plot for two gases (assuming ideal) during adiabatic processes are given in the figure. Plot A and Plot B should correspond respectively to:
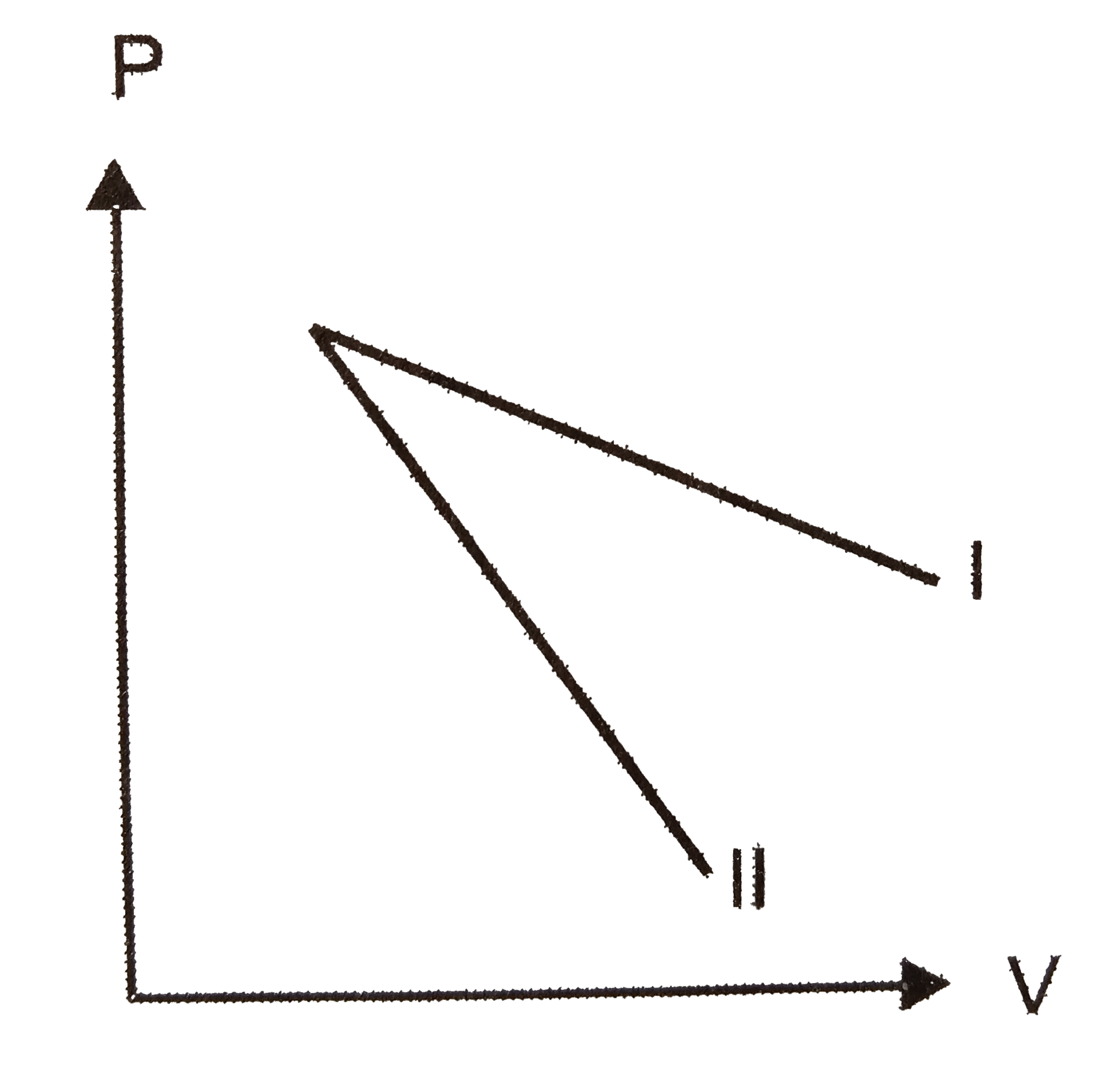
View SolutionP−V plots for two gases during adiabatic expansion are shown in figure. Plot I and II should correspond respectively to :
View SolutionP-V plots for two gases during adiabatic processes are shown in the figure. Plots 1 and 2 should corresponds respectively to

View SolutionP-V plots for two gases during an adiabatic process are given in the figure:
Plot A and plot B should correspond to : (Assume ideal behaviour)

View Solution