ΔUfor a system that does 1000 cals of work on the surroundings when 200 cals of heat are absorbed by the system is
- 1200 cals
+ 1200 cals
+ 800 cals
- 800 cals
The correct Answer is:D
ΔU=200−1000=−800 cals.
Similar Questions
If 500 calorie of heat energy are added to a system and the system does 350 calorie of work on the surroundings, what is the energy change of the system?
View SolutionIn a process, a system does 145 J of work on its surroundings while 85 J of heat is added to the system. What is ΔU for the system?
View Solution
Knowledge Check
ΔU for a system that does 1000 cal of work on the surroundings when 200 cal of heat are absorbed by the system is ______ .
A−800 calB+800 calC−1200 calD+1200 calWhat is ΔE for system that does 500 cal of work on surrounding and 300 cal of heat is absorbed by the system ?
A−200calB−300 calC+200 calD+300 calwhat is ΔE for a system that does 500 cal of work pm surrounding and 300 cal of heat is adsorbed by the system ?
A−200calB−300calC+200calD+300cal
If "10" calorie of heat energy is added to a system and the system does "5" calorie of work on the surroundings,What is the energy change of the system?
View SolutionA system is taken from state a to state c along the path adc (figure). The amount of heat absorbed is 10cals and work done on the gas is 138J. Calculate the work done on//by the gas when 20cals of heat are absorbed in taking the system from state a to state c along the path abc
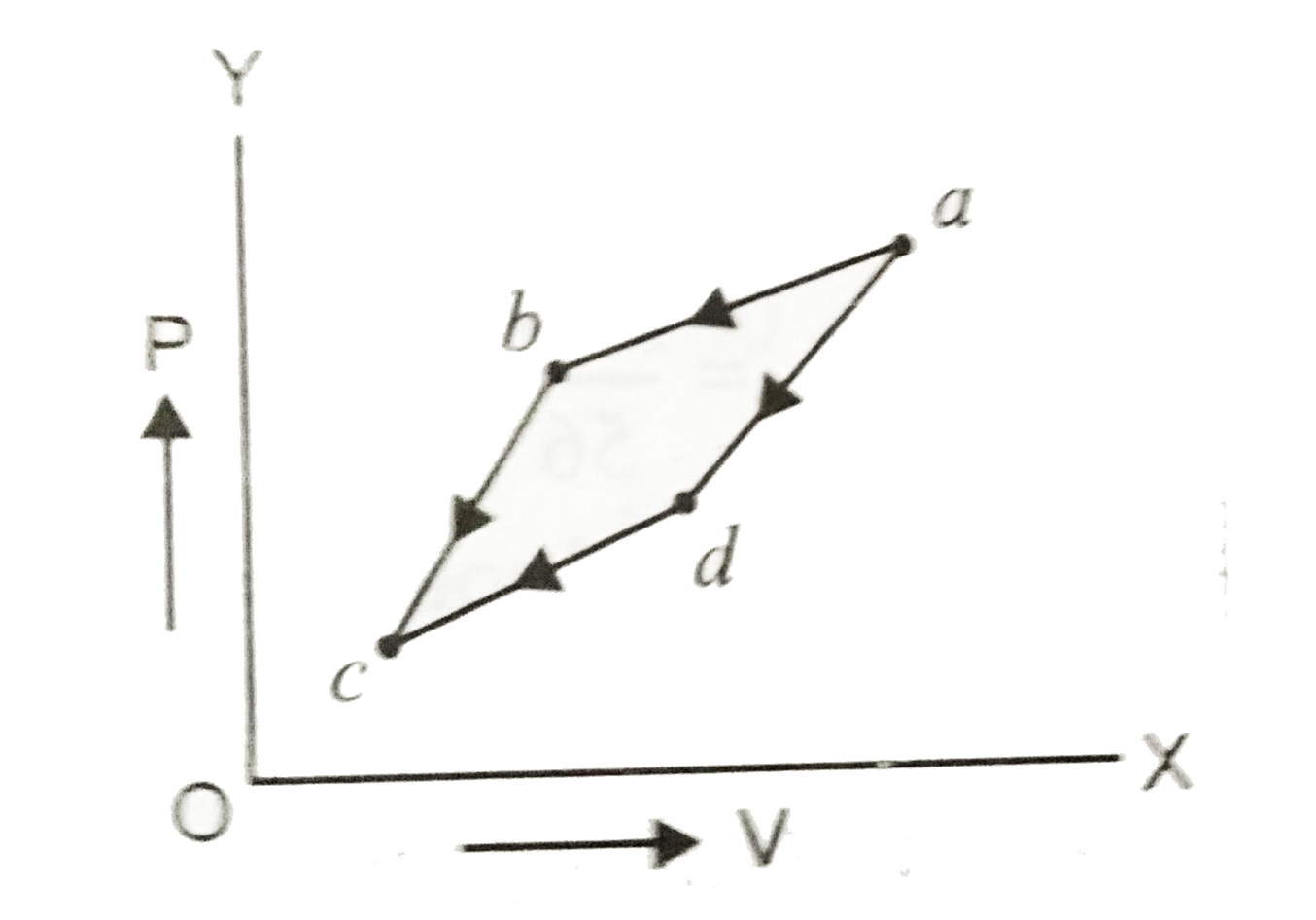
View SolutionIn a process, a system does 140 J of work on the surroundings and only 40 J of heat is added to the system, hence change in internal energy is
View SolutionIf 50 calories are added to a system and system does work of 30 calories on surroundings, the change in internal energy of system is
View SolutionWork done by the system on surroundings is :
View Solution

