ΔU=+200J for a system that gives off 200 J of heat and
does 200 J of work
has 400 J of work done on it
does no work
has 100 J of work done on it
The correct Answer is:B
ΔU==200j,q=−200j
ΔU=q+W⇒∴W=ΔU−q
W=+200J,==400J
Similar Questions
Knowledge Check
ΔU for a system that does 1000 cal of work on the surroundings when 200 cal of heat are absorbed by the system is ______ .
A−800 calB+800 calC−1200 calD+1200 calA system has internal energy equal to U. 600 J of work is done on the system when 450 J of heat is lost. The final energy of system is
AU + 1050 JBU - 150 JCU + 150 JDU-1050 JA thermodynamic process is shown in figure. In process ab, 600 J of heat is added, and in process bd 200 J of heat is added. The total heat added in process acd is
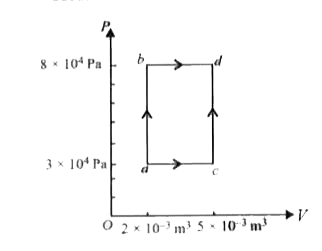 A550 JB650 JC750 JD850 J
A550 JB650 JC750 JD850 J
For a gaseous system, if heat rejected by the system is 100J and work done by the system is 20J , then change in internal energy is
View SolutionCalculate the changes in internal energy of a system if,
300 J of heat energy is added to the system and a work of 160 J is done on the system.
View SolutionWhat is the efficiency of a machine if, of the 1000 J of work done by the effort, 200 J are done in overcoming friction?
View Solution(a) An ideal gas contained in a cylinder is given 200 J of heat. The gas does 60 J of work in the expansion resulting from heating. Calculate the increase in internal energy in the process.
(b) Calculate the change in internal energy of a gas kept in a rigid container when 150 J of heat is supplied to it.
View SolutionA system absorbs 800 J of heat and system works equivalent to 900 J by expanding. The value of ΔU for the system is
View Solution
