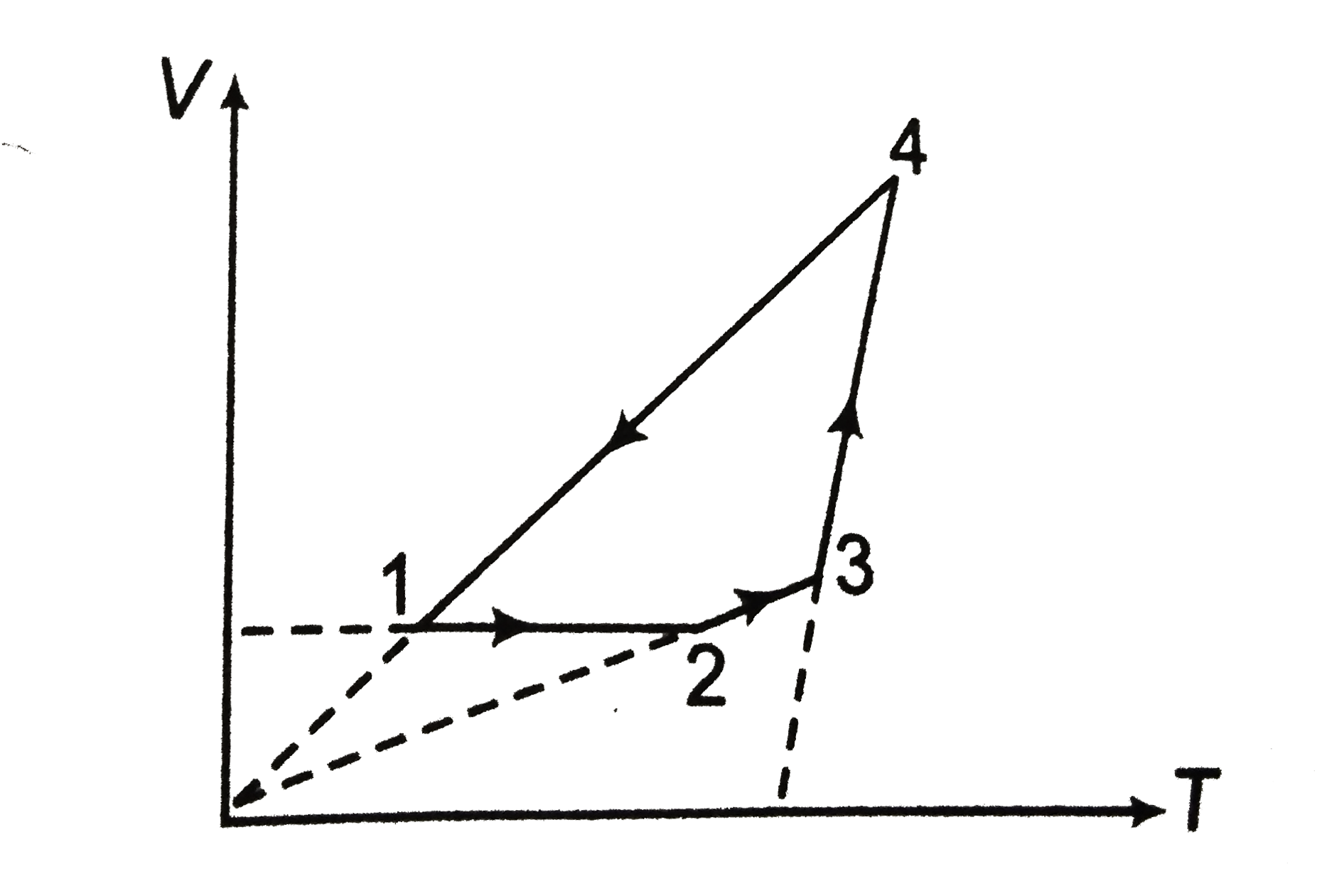
V-T diagram for n mol monoatomic gas is given below
_PHY_E01_011_Q01.png)
Choose the correct statement"
A
∣∣∣ΔQ1→2ΔQ3→4∣∣∣=12
B
∣∣∣ΔQ1→2ΔQ2→3∣∣∣=53
C
Work done in cyclic process is ΔW=nRT02
D
There are only adiabatic and isochoric processes are involved
Video Solution
Text Solution
The correct Answer is:B, C
_PHY_E01_011_S01.png)
(A)∣∣∣ΔQ1→2ΔQ3→4∣∣∣=∣∣∣NCPΔT1→2NCPΔT3→4∣∣∣=T0T0/2=2
(B) ∣∣∣ΔQ1→2ΔQ2→3∣∣∣=∣∣∣NCPΔT1→2NCVΔT2→3∣∣∣=CPCV=53
(C) WCycle=P0V0=nR[T02][Using point no. 4]
(D) Wrong as no adiabatic process is involved.
|
Similar Questions
Knowledge Check
Circuit as shown in figure below, choose the correct statement
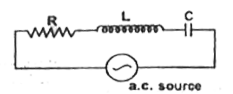 A(A) current in resistance R and current in inductor L.a.c. source will be in 90^(@ phase differenceBotential drop across R and potential drop across L will be in same phaseCcurrent through C and current through L will be in 90∘ phase differenceDcurrent in R and current in L will be in same phase
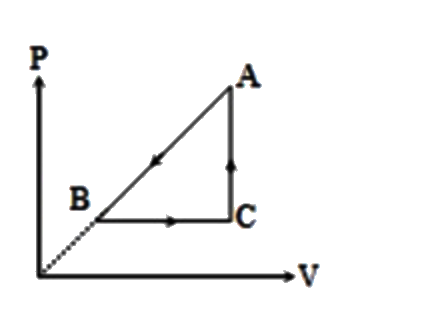
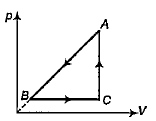
A(A) current in resistance R and current in inductor L.a.c. source will be in 90^(@ phase differenceBotential drop across R and potential drop across L will be in same phaseCcurrent through C and current through L will be in 90∘ phase differenceDcurrent in R and current in L will be in same phaseA cyclic process of an ideal monoatomic gas is shown in figure. The correct statement is (are):
 AWork done by gas in process AB is more than that of the process BC.Bnet heat energy has been supplied to the system.Ctemperature of the gas is maximum in state BDin process CA, heat energy is rejected out by system.
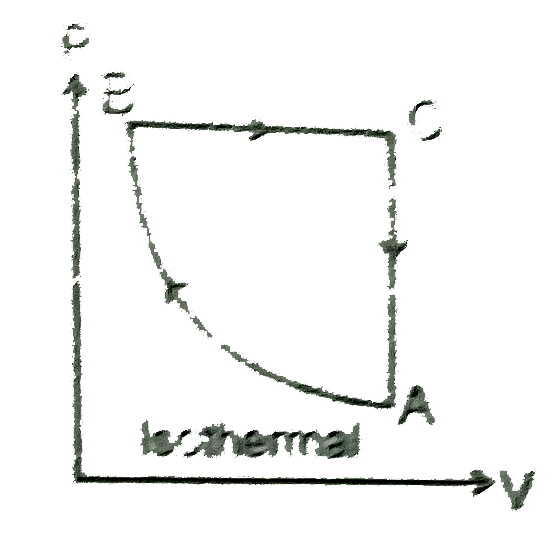
AWork done by gas in process AB is more than that of the process BC.Bnet heat energy has been supplied to the system.Ctemperature of the gas is maximum in state BDin process CA, heat energy is rejected out by system.. p-V diagram of a cyclic process ABCA is as shown in figure. Choose the correct statement
 A△QA→B=constantB△UB→C=positiveC△UCAB=negativeDAll of the above
A△QA→B=constantB△UB→C=positiveC△UCAB=negativeDAll of the above
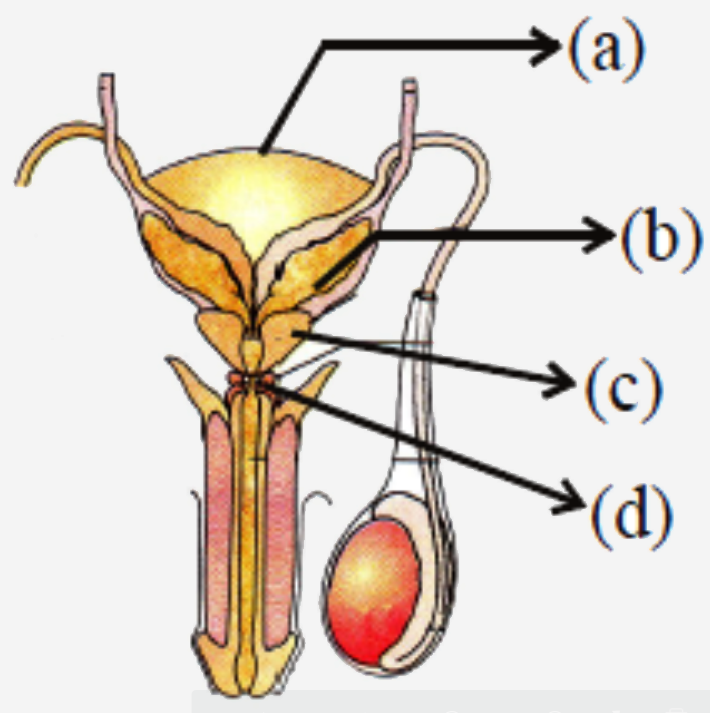
Consider the diagram given below and choose the correct option.
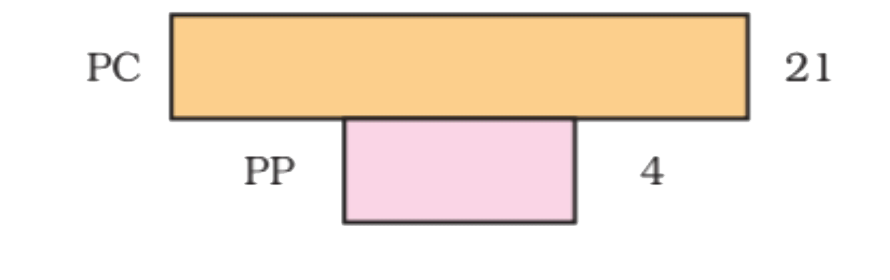
View SolutionBased on the diagram given below , choose the correct statement from the following .
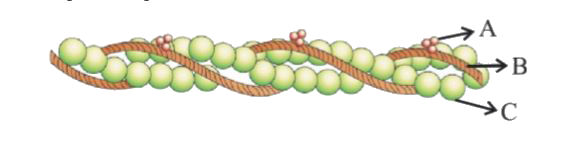
View SolutionOn the basis of below given diagram select the incorrect statement -
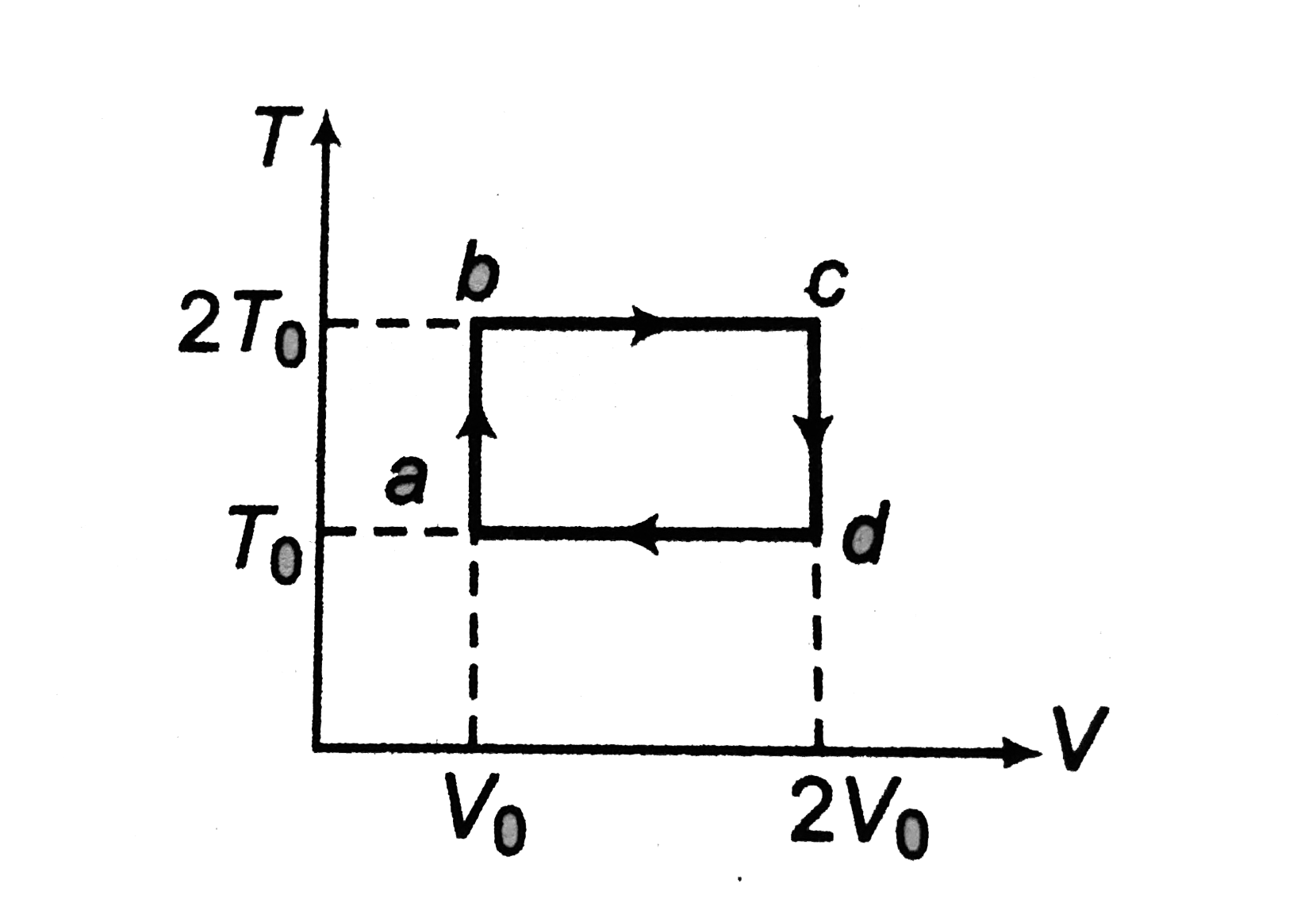
View SolutionT-V diagram of two moles of a monoatomic gas is as shown in figure.
For the process abcda choose the correct options given below
View SolutionA cyclic process 1-2-3-4-1 is depicted on V-T diagram. The p-T and p-V diagrams for this cyclic process are given below. Select the correct choices (more than one options is/are correct)
View Solution