Choose the correct option that gives aromatic compound as major product:
A
_CHM_E01_002_O01.png)
B
_CHM_E01_002_O02.png)
C
_CHM_E01_002_O03.png)
D
_CHM_E01_002_O04.png)
Video Solution
Text Solution
The correct Answer is:A, B
|
Similar Questions
Knowledge Check
Choose the correct options:
AFavourable conditions for formation of graphite are high pressure and low temperature from equilibrium diamond (d=3.5g/ml)⇔ graphite (d=2.3g/ml),Δ=−1.9kJ/moleBFor reaction N2O4(g)⇔2NO,(g), degree of dissociation (α) is √K4P+KP where P is equilibrium pressure.CFor reaction CI2(g)+3F2(g)⇔2CIF3(g),ΔH=−329kJ ,dissociation of CIF3(g) will be favoured by additon of inert gas at constant pressure.DReaction stops at equilibrium (microscopically).Choose the correct option(s) that give(s) an aromatic compound as the major product.
A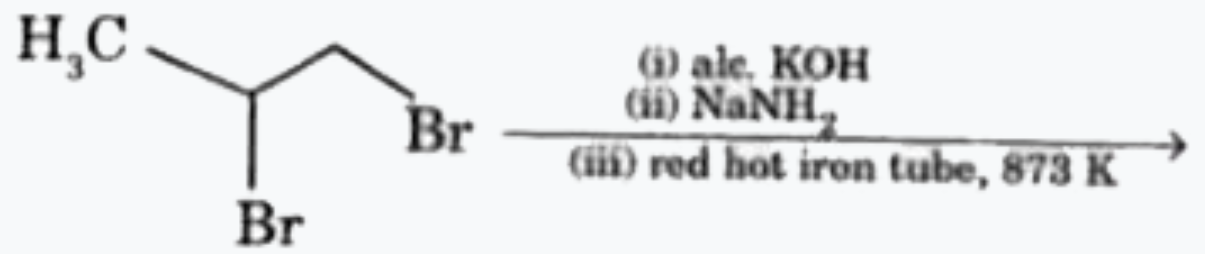 B
B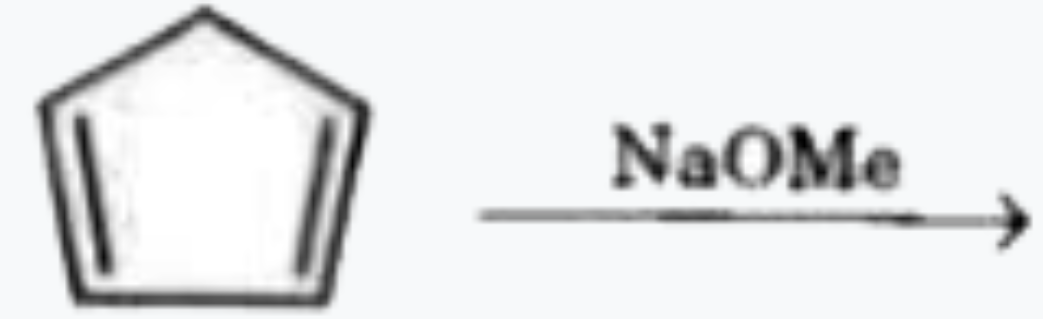 C
C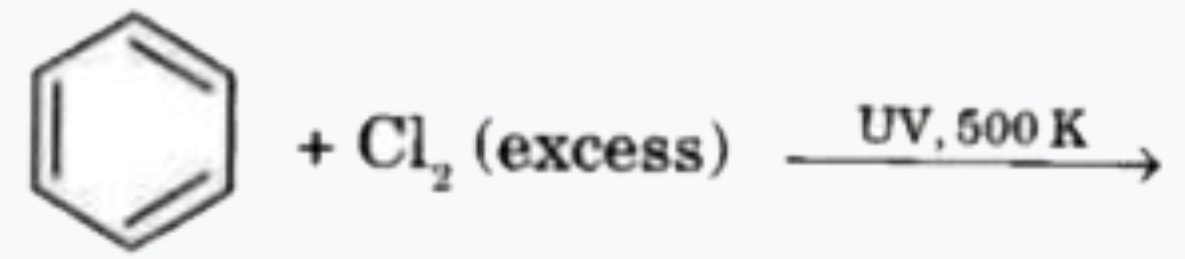 D
D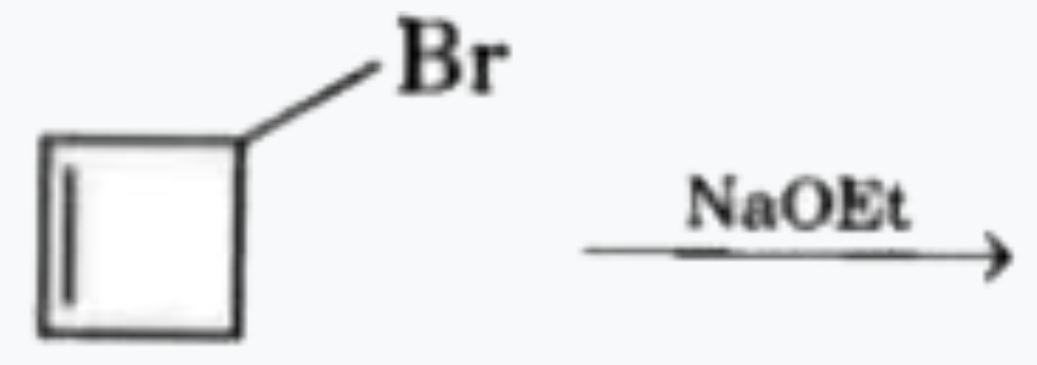
Choose the correct option(s) that give(s) an aromatic compound as the major product.
A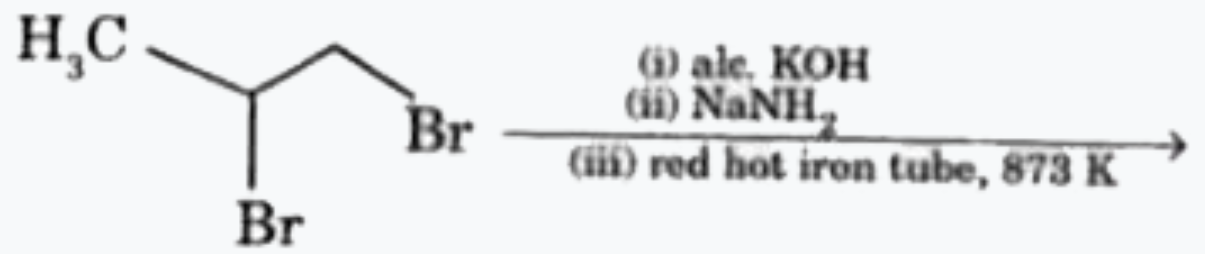 B
B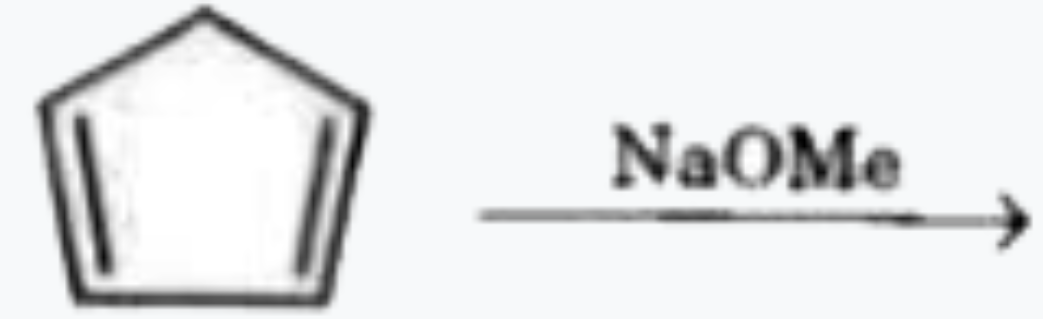 C
C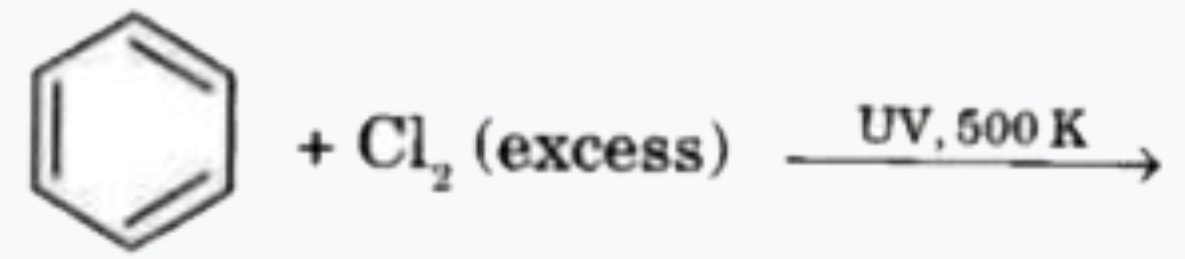 D
D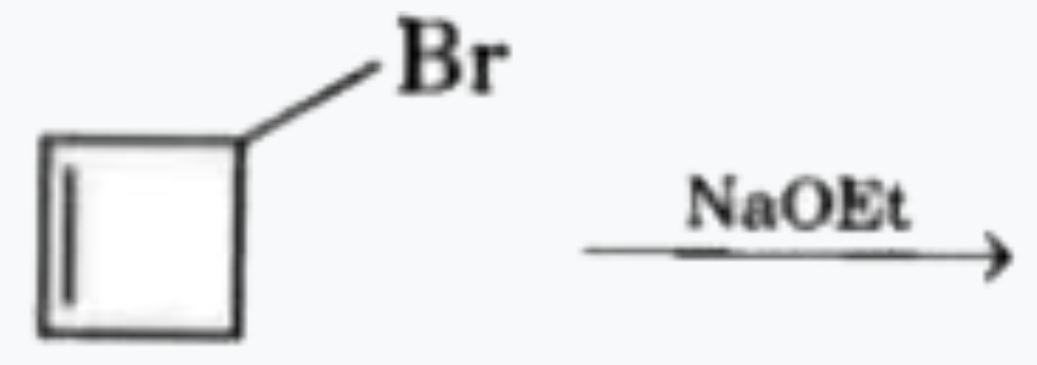
Choose the correct option(s) that give(s) an aromatic compound as the major product
View SolutionChoose the correct option(s) that give(s) an aromatic compound as the major product
View SolutionChoose the correct option for major product
View SolutionChoose the compounds below that are aromatic :
View SolutionChoose the correct option regarding compound microscope:
View Solution


_CHM_E01_002_S01.png)