The relative reactivities of acyl compound towards nucleophilic substitution are in the order of
A
ester gt acyl chloride gt amide gt acid anhydride
B
acid anhydride gt amide gt ester gt acyl chloride
C
acyl chloride gt ester gt acid anhydride gt amide
D
acyl chyl chloride gt acid anhydride gt ester gt amide
Video Solution
Text Solution
The correct Answer is:D
Batter is leaving gp, higher will be reactivity of acyl compound toward towards nucleophile acyl substitution. Weaker is the base, better is leaving gp. Stronger is base, weaker is its acid and vice-versa.
|
Similar Questions
Knowledge Check
The relative reactivities of acyl compounds towards nucleophilic substitution are in the order of
AAcid anhydride > Amide > Ester > Acyl chlorideBAcyl chloride > Ester > Acid anhydride > AmideCAcyl chloride > Acid anhydride > Ester > AmideDEster > Acyl chloride > Amide > Acid anhydrideThe relative reactivities of acyl compounds towards nucleophilic substitution are in the order of
Aacid anhydride gt amide gt ester gt acyl chlorideBacyl chloride gt ester gt acid anhydride gt amideCacyl chloride gt acid anhydride gt ester gt amideDester gt acyl chloride gt amide gt acid anhydrideThe relative reactivities of acyl compound towards nucleophilic substitution are in the order of
AAcid anhydride > Amide > Ester > Acyl chlorideBAcyl chloride > Ester > Acid anhydride > AmideCAcyl chloride > Acid anhydride > Ester > AmideDEster > Acyl chloride > Amide > Acid anhydride
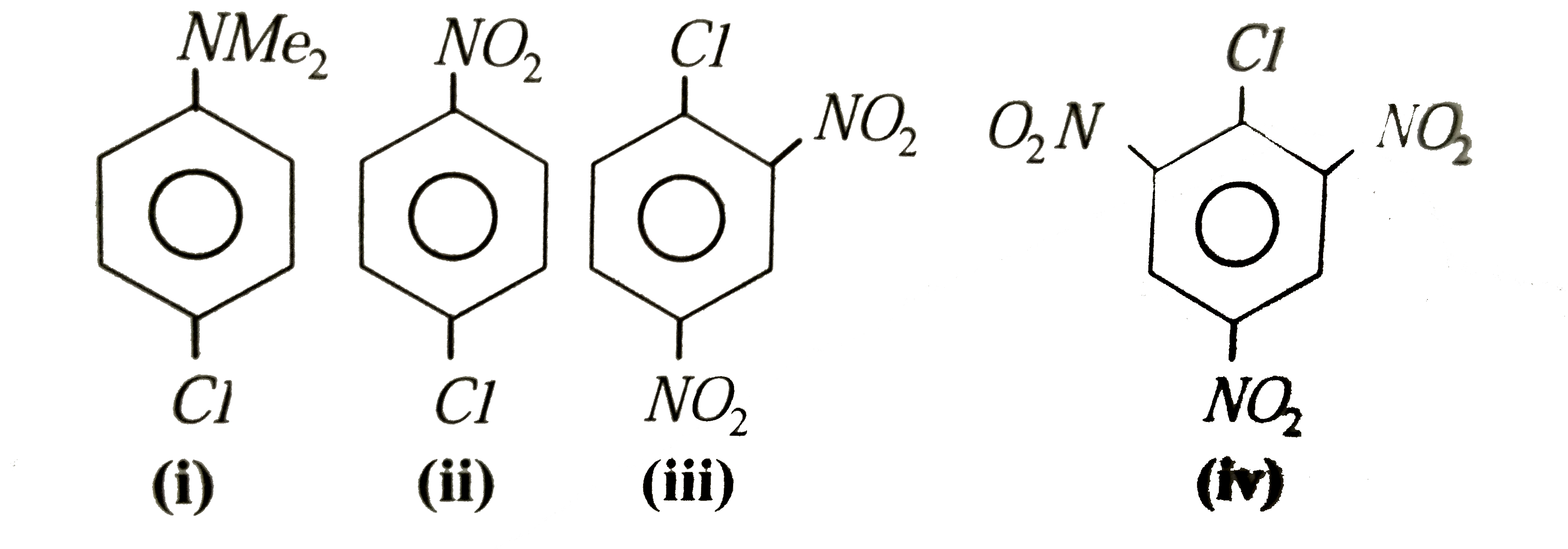
The decreasing oder of reactivity of given compound towards nucleophilic substitution with aqueous NaOH is :
(I)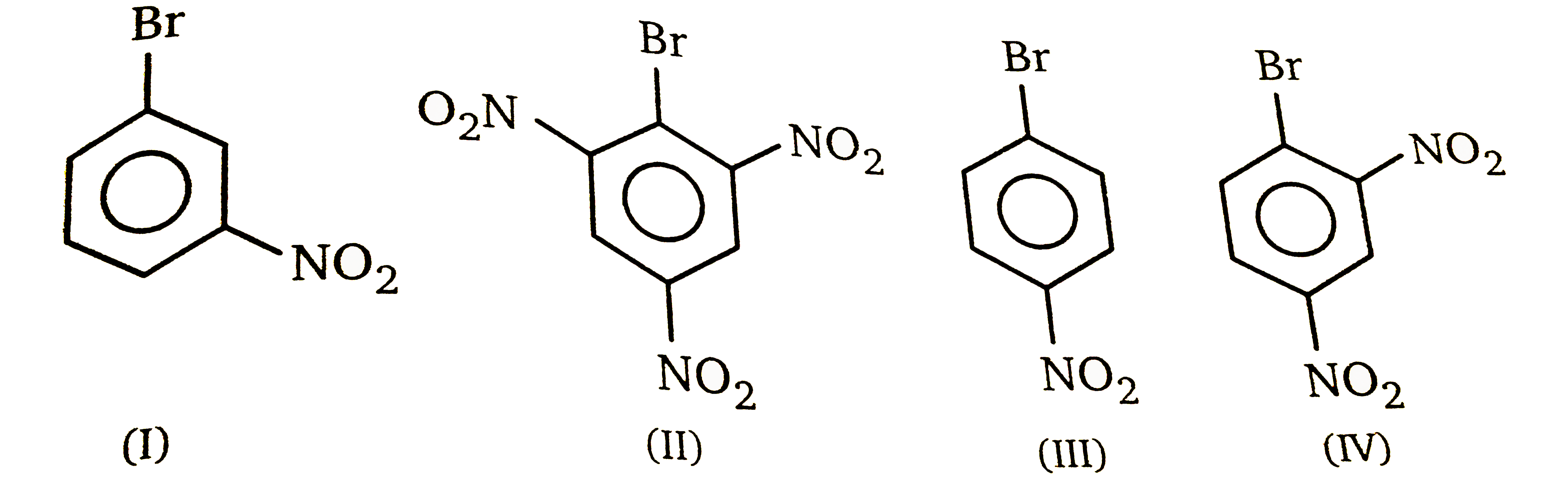
View SolutionThe correct order of the reactivity of the following compounds towards nucleophilic substitution in the following compounds is
 .
.
View SolutionConsider the following alcohols,
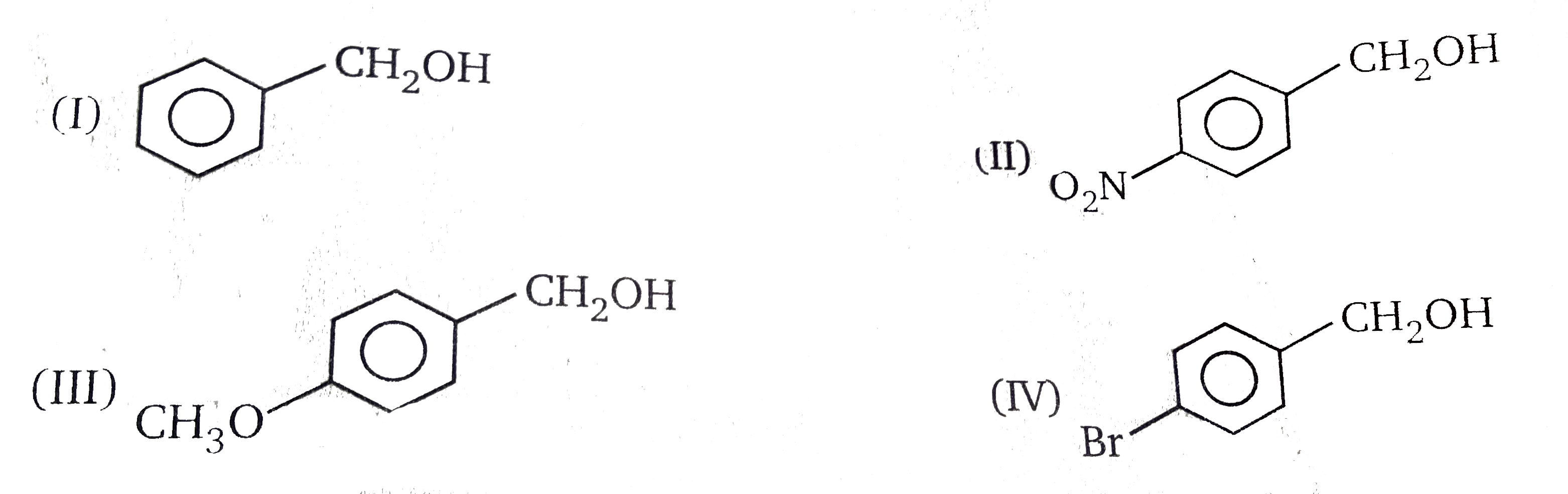
The order of decreasing reactivities of these alcohols towards nucleophilic substitution with HBr is :
View SolutionThe most reactive compound towards electrophilic substitution reaction is
View SolutionThe most reactive compound towards electrophilic substitution reaction is :-
View Solution