Tertiary nitro compounds cannot show tautomerism becauses :
A
They are very stable
B
They isomerise to give secondary nitro compound
C
They do not have labile hydrogen atom.
D
They are highly reactive.
Video Solution
Text Solution
The correct Answer is:C
Nitroalkane containing α-H atoms, i.e., 1∘ and 2∘ nitroalkanes show tautomerism.
|
Similar Questions
Knowledge Check
Tertiary nitro compounds do not tautomerise because
Athere is no double bondBthere is no alpha-hydrogenCoxygen is more electronegative than HDAll of the aboveWhich cannot show tautomerism ?
A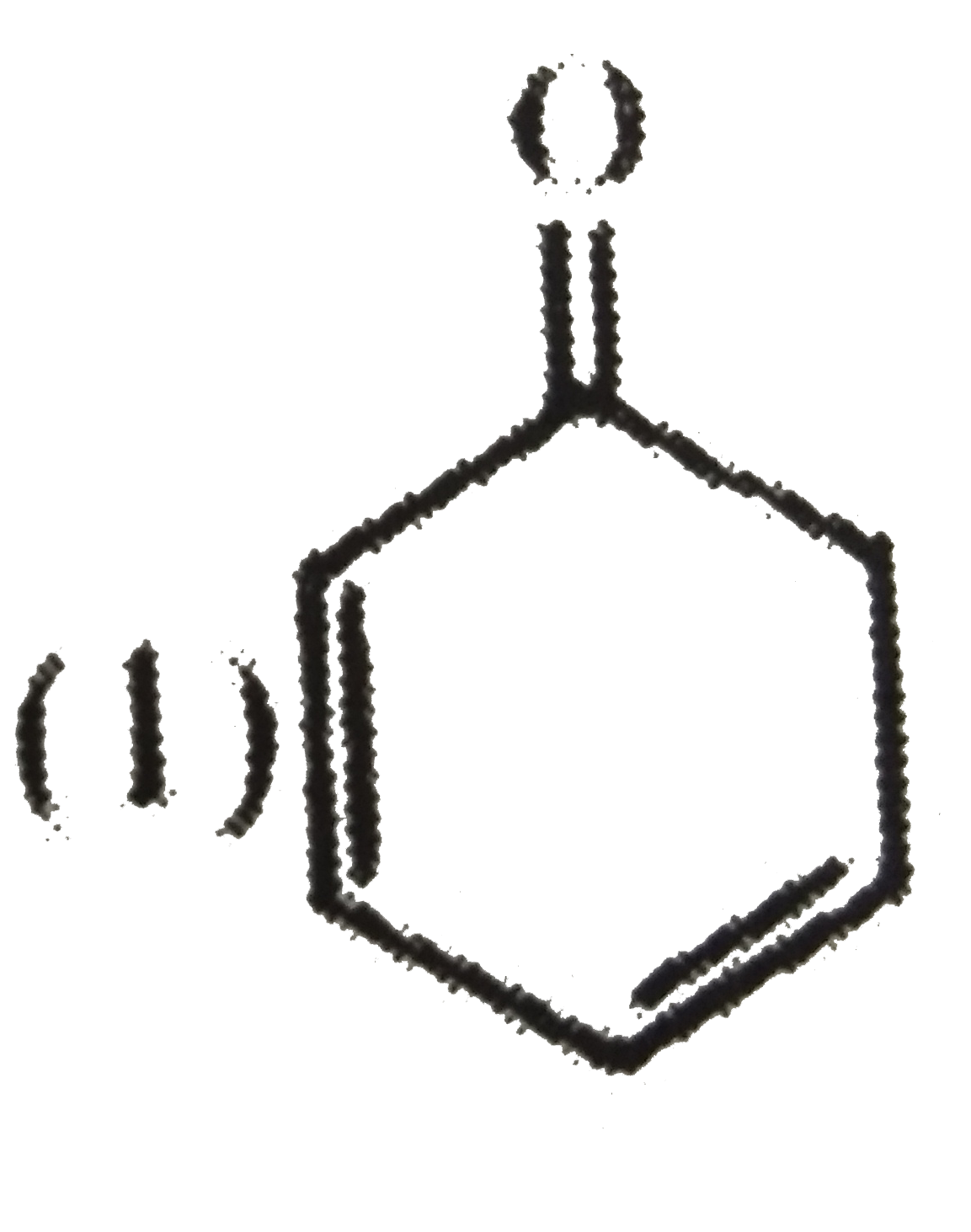 B
B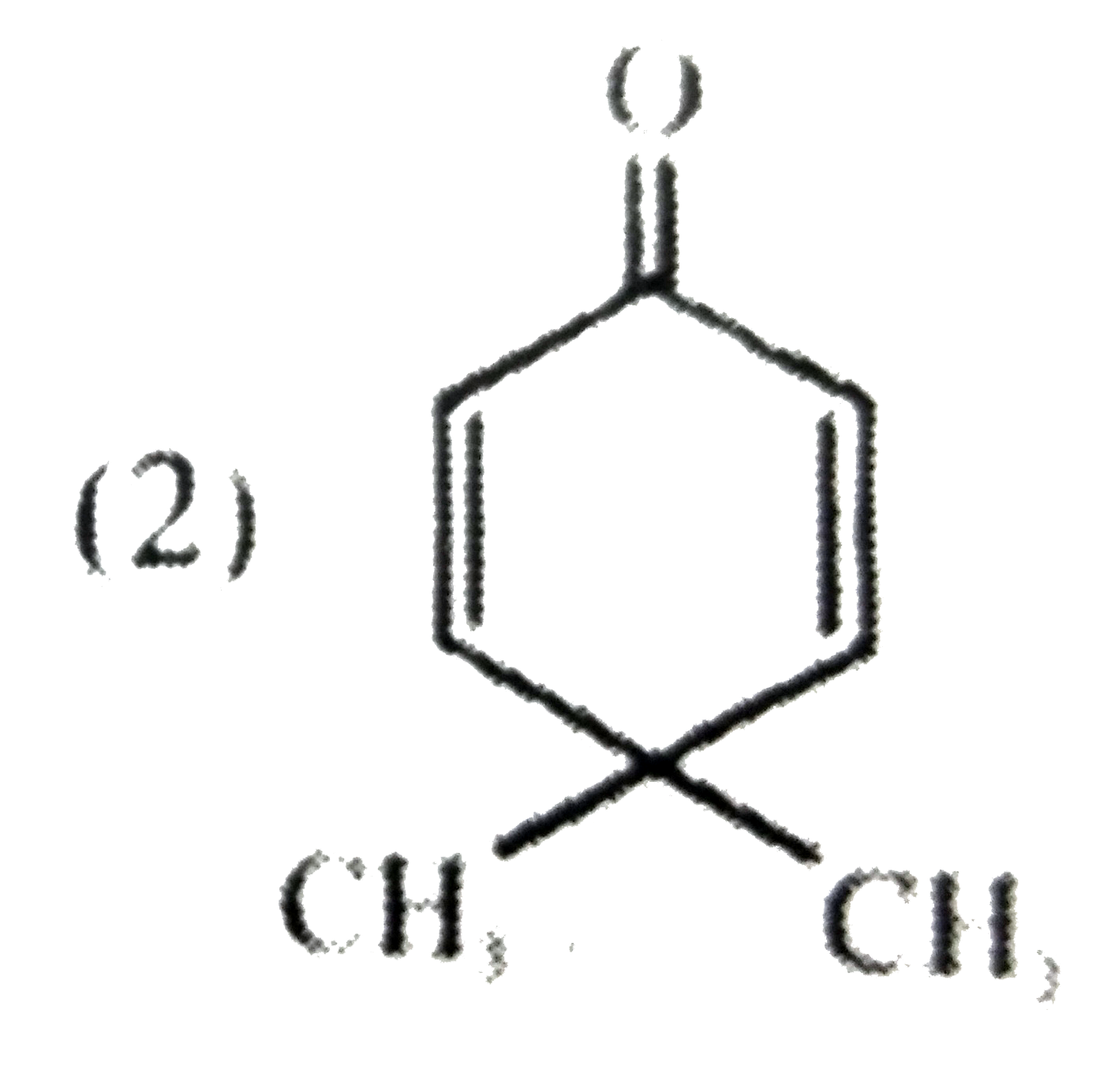 C
C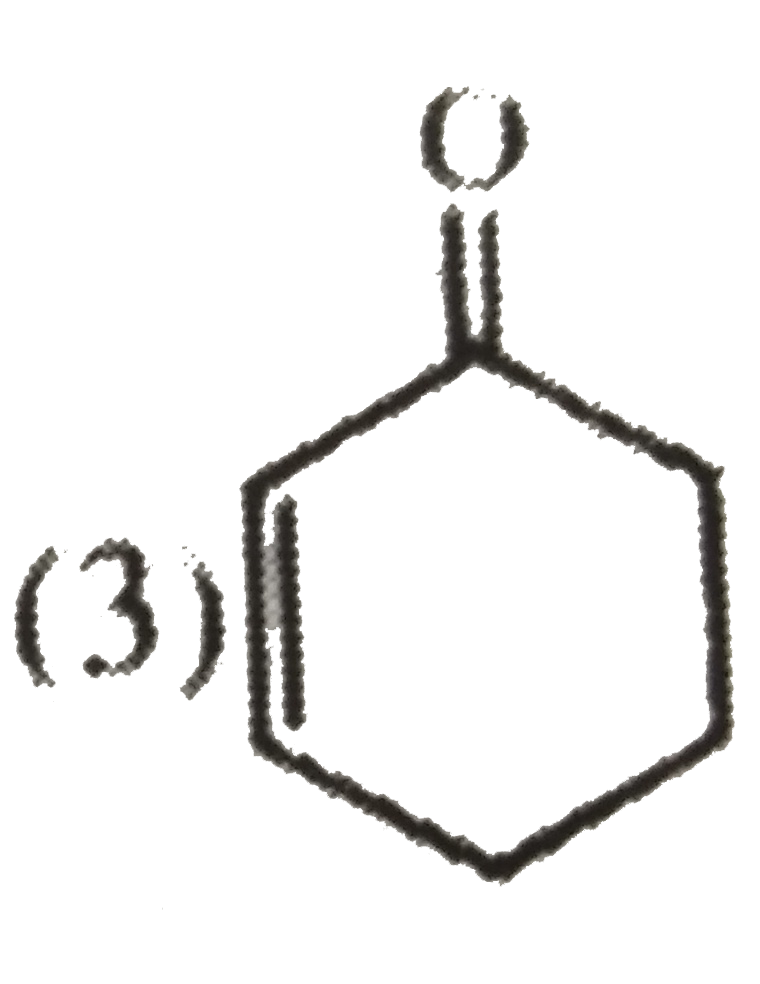 D
D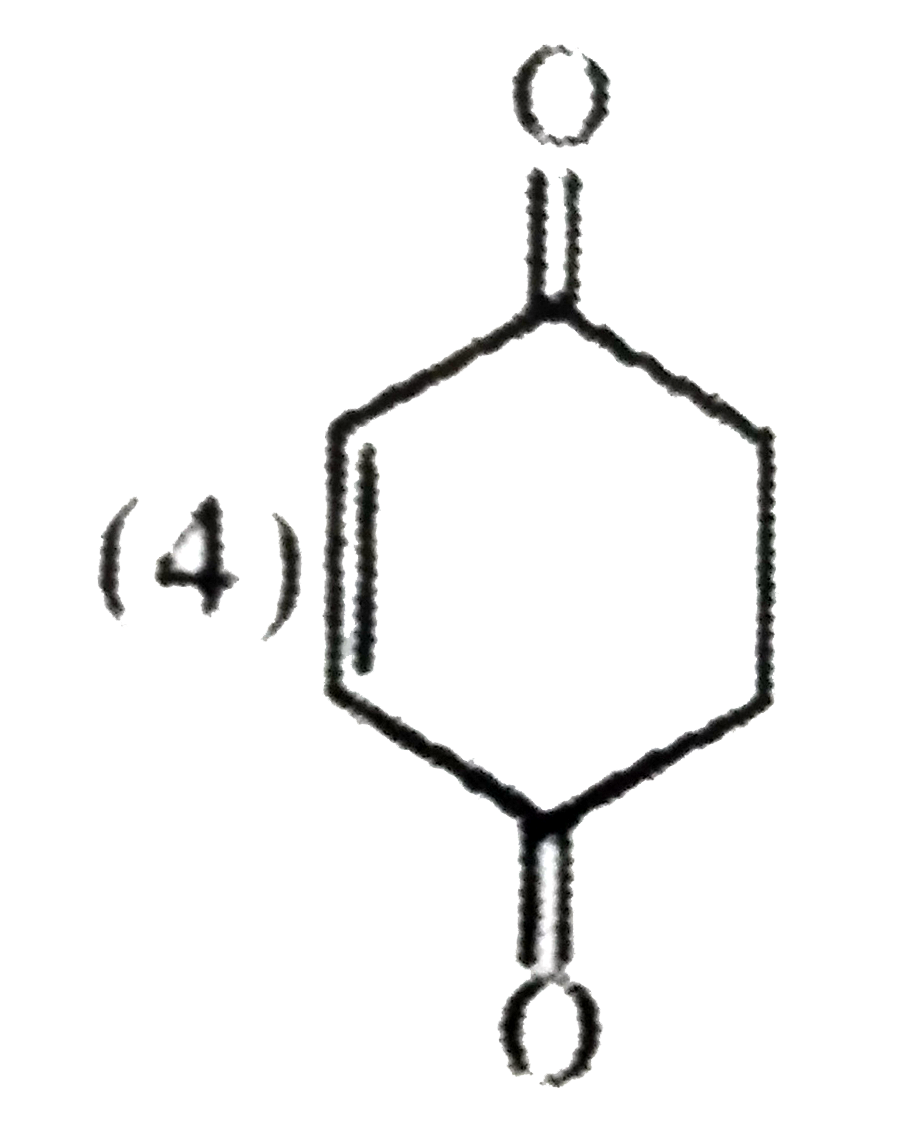
Which can show tautomerism?
A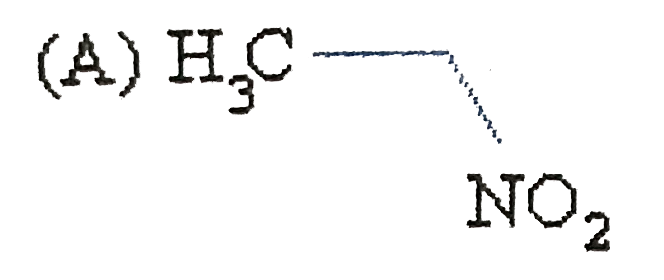 B
B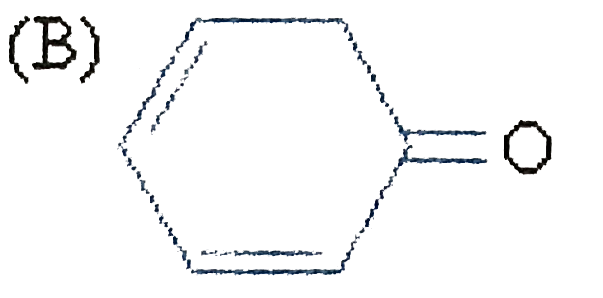 C
C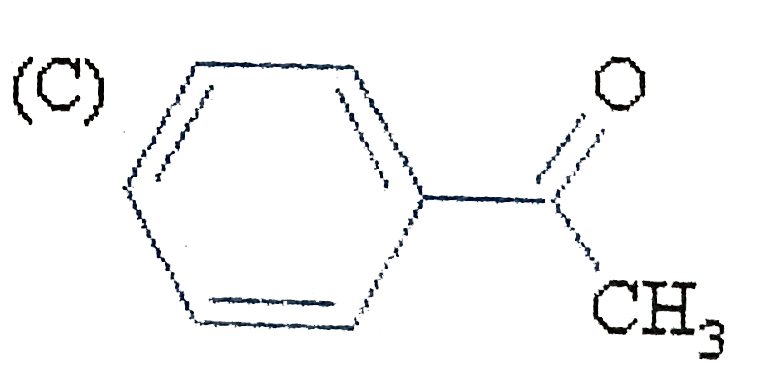 DHCN
DHCN
Which compound show tautomerism :
View SolutionTotal number of compound which can shows tautomerism are :-
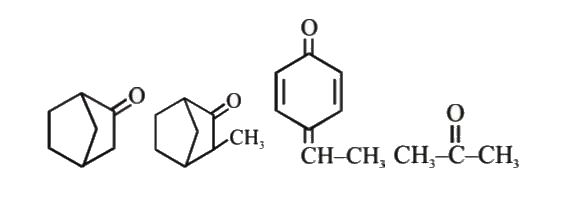
View SolutionWhich one of the following compounds cannot show tautomerism?
View SolutionTertiary nitroalkane cannot tautomerise because
View SolutionWhich one of the following compounds cannot show tautomerism?
View Solution

