The boiling point of a compound is raised by _________.
intramolecular hydrogen bonding
intermolecular hydrogen bonding
covalent bonding
ionic covalent
The correct Answer is:B
Step by step video, text & image solution for The boiling point of a compound is raised by _________. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The boiling point of a compound does not depend upon
ASolubility of the compound in waterBHydrogen bondingCSize of the moleculeDPolarity of the molecule.Assertion: The boiling point of the compounds increases in the order:
Isopropylchloridelt1-Chloropropane,1-Chlorobutane. ltbr> Reason: Boiling point depends upon the molecular mass and surface area.AIf both assertion and reason are true and reason Is the correct explanation of assertion.BIf both assertion and reason are true but reason is not the correct explanation of assertion.CIf assetion is true but reason is false.DIf both assertion and reason are false.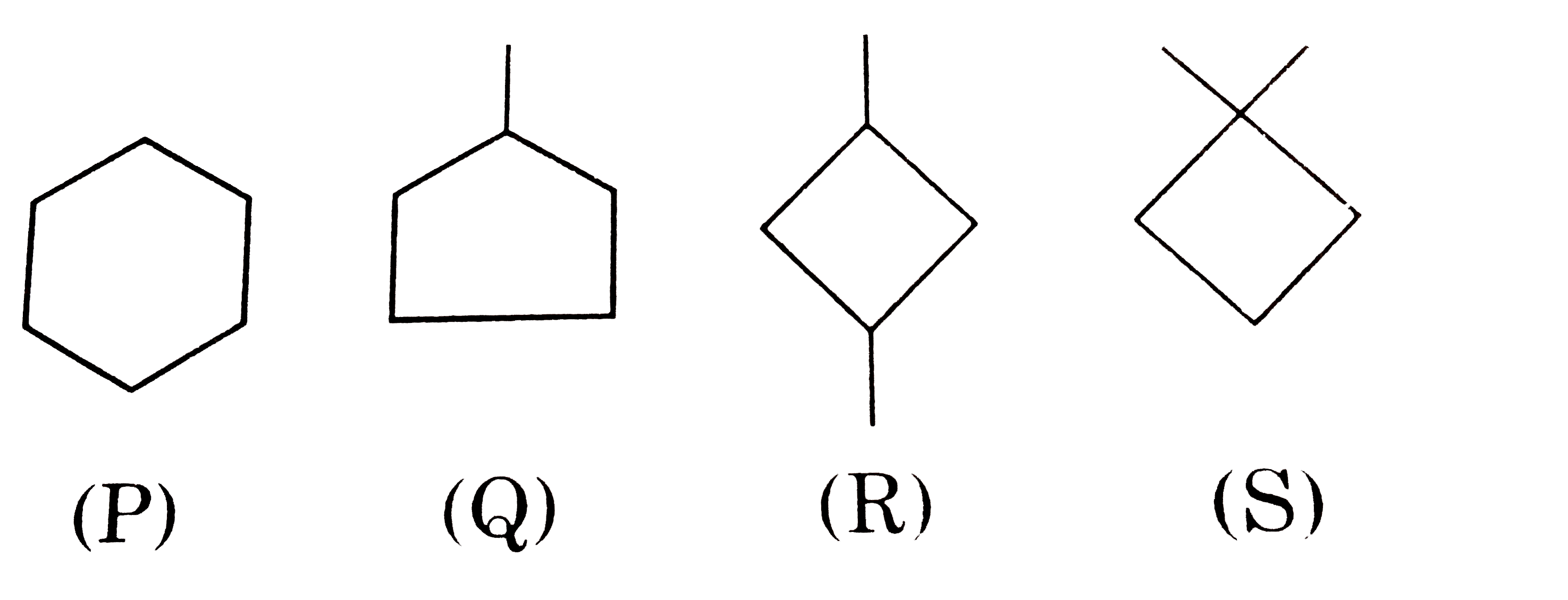
Correct order of boiling points of the above compound is :AP>Q>R>SBP>Q>S>RCR>S>Q>PDS>R>Q>P
Generally the melting and boiling points of carbon compounds are high. True/false
View SolutionWhy is boiling point of the solution gets raised when a non-volatile solute is added to it?
View SolutionHydrogen bonds are formed in many compounds e.g. H2O, HF, NH3. The boiling point of such compounds depends to a extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points above compounds is
View SolutionHydrogen bonds are formed in many compounds e.g. H2O, HF, NH3. The boiling point of such compounds depends to a extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points above compounds is
View SolutionLowest boiling point will be of the compound-
View Solution

