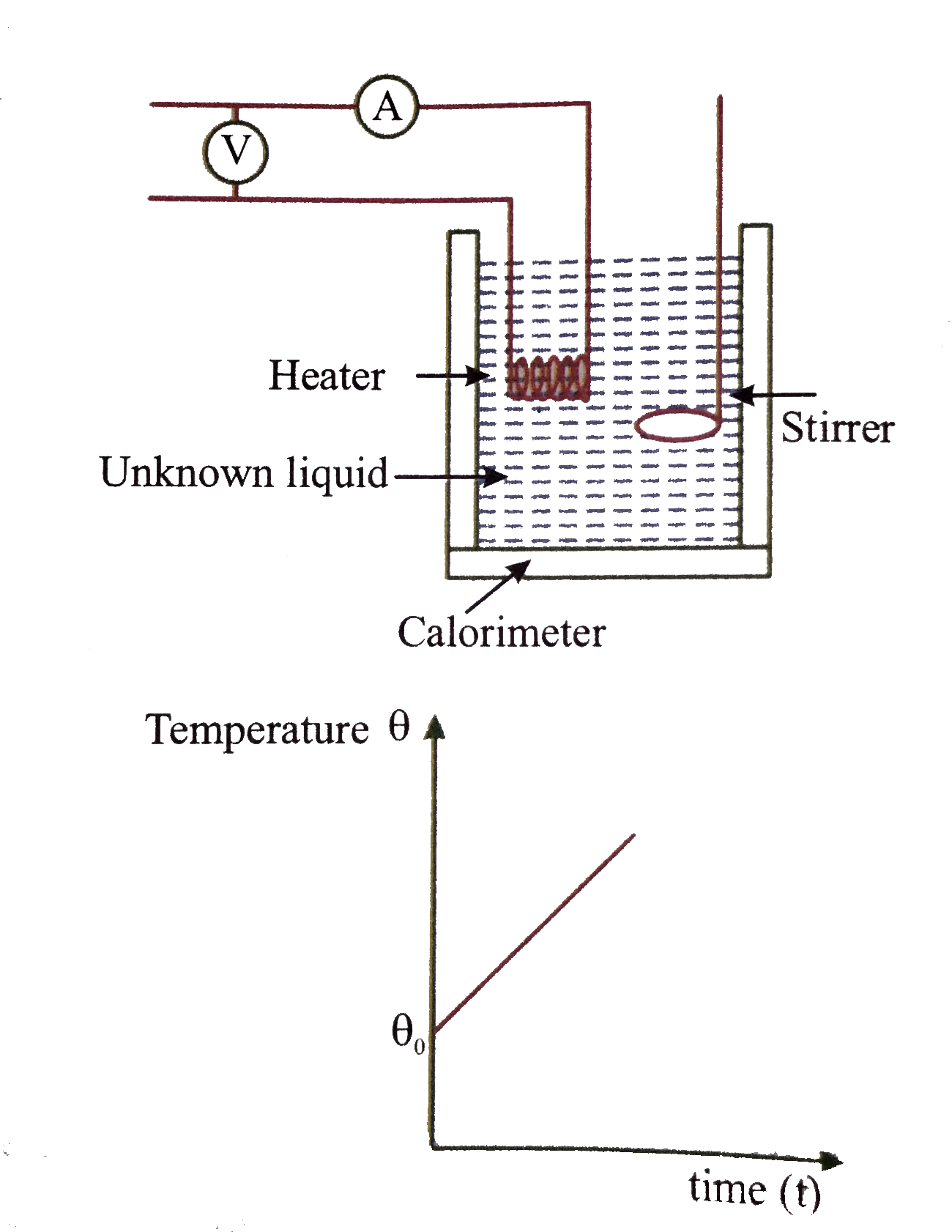
Figure shows an electrical calorimeter to determine specific heat capacity of an unknown liquid, First of all, the mass of empty calorimeter (a copper container) is measured and suppose it is m1. Then the unknown liquid is poured in it. Now the combined mass of calorimeter+liquid system is measured and let it be m2. So the mass of liquid is (m2−m1). Initially both were at room temperature (θ0).
Now a heater is immeresed in if for time interval t. The voltage drop across the heater is V and current passing through it is I. Due to heat supplied, the temperature of both the liquid and calorimeter will rise simultaneously. After tsec, heater was switched off, and final temperature is θr. If there is no heat loss to surroundings. Heat supplied by the heater=Heat absorbed by the liquid+heat absorbed by the calorimeter (VI)t=(m2−m1)S1(θf−θ0)+m1Sc(θf−θ0)
The specific heat of the liquid S1=(VI)tθf−θ0−m1Sc(m2−m1)
Radiation correction: There can be heat loss to environment. To compensate this loss, a correction is introduced.
Let the heater was on for tsec, and then it is switched off. Now the temperature of the mixture falls due to heat loss to environment. The temperature of the mixture is measured t/2sec. after switching off. Let the fall in temperature during this time is varepsilon
Now the corrected final temperature is taken as θf=θf+ɛ
If mass and specific heat capacity of calorimeter is negligible, what would be maximum permissible error in Sl. Use the date mentioned below.
m1→, Sl→0, m2=1.00kg,
V=10.0V, I=10.0A,
t=1.00×102sec, θ0=15∘C,
Corrected θ1=65∘C
4%
5%
8%
12%
The correct Answer is:C
If m1→0, Sl→0, Sl=VItm2(θf−θ0)
dSlSl=ΔVV+ΔII+Δtt+Δm2m2+Δθr+Δθ0θr−θ0
=0.110.0+0.110.0+0.01×1021.00×102+0.011.00+1+150=8%
Similar Questions
Two sphere of massM1 areM2 situated in air and the gravitational force between them is F. The space around the masses is now filled with liquid of specific gravity 3. The gravitational force will now be.........
View SolutionTwo liquids of masses M_(1) , and M_(2) and specific heats S_(1) , and S_(2) , respectively are mixed. The specific heat of the mixtureis
View SolutionThe mass, specific heat capacity and the temperature of a solid are 1000g,(1/2) ((cal)/g) and 80 degrees C respectively. The mass of the liquid and the calorimeter are 900g and 200g. Initially, both are at room temperature 20 degrees C. Both calorimeter and the solid are made of the same material. In the steady state, temperature of mixture is 40 degrees C, then specific heat capacity of the unknown liquid is;
View SolutionIn electrical calorimeter experiment, voltage across the heater is 100.0V and current is 10.0A. Heater is switched on for t =700.0s. Room temperature is θ0=10.0∘C and final temperature of calorimeter and unknown liquid is θf=73.0∘C. Mass of empty calorimeter is m1=1.0kg and combined mass of calorimeter and unknown liquid is m2=3.0kg. Find the specificheat capacity of the unknown liquid in proper significant figures. Specific heat of calorimerter = 3.0×103j/kg.∘C.
View SolutionTwo blocks of masses m_(1) and m_(2) are kept on a smooth horizontal table as shown in figure. Block of mass m_(1) (but not m_(2) is fastened to the spring. If now both the blocks are pushed to the left so that the spring is compressed a distance d. The amplitude of oscillatin of block of mass m_(1) after the system is released is
View SolutionAssertion : Mass of solid floating in liquid is m_(1) and mass of liquid is m_(2) . Base area is A. Then pressure at bottom is p_(0) + ((m_(1)+m_(2))g)/(A) Reason : Upward force on liquid from base of vessel is pA, where p is pressure at bottom.
View Solutionएक तांबे के एक कैलोरीमीटर जिसका द्रव्यमान m1= 1kg है। पानी जिसका द्रव्यमान m2=1 kg है। इसको इस कैलोरीमीटर में भरा गया है। उनका उभयनिष्ठ तापमान t = 10°C है। अब बर्फ का टुकड़ा जिसका द्रव्यमान m3=2 kg तथा –11°C ताप वाले इस टुकड़े को कैलोरीमीटर में गिराया जाता है। किसी भी प्रकार की ऊष्मा हानि नगण्य मानो। निकाय का अन्तिम तापमान है - [ताँबे की विशिष्ट ऊष्मा =0.1 Kcal/ kg°C, पानी की विशिष्ट ऊष्मा =1 Kcal/kg°C, बर्फ की विशिष्ट ऊष्मा =0.5 Kcal/kg°C, बर्फ के गलन की गुप्त ऊष्मा =78.7 Kcal/kg]
View SolutionWater of mass m2=1kg is contained in a copper calorimeter of mass m1=1kg. Their common temperature t=10∘C. Now a piece of ice of mass m2=2kg and temperature is −11∘C dropped into the calorimeter. Neglecting any heat loss, the final temperature of system is. [specific heat of copper =0.1Kcal/kg∘C, specific heat of water =1Kcal/kg∘C, specific heat of ice =0.5Kcal/kg∘C, latent heat of fusion of ice =78.7Kcal/kg]
View Solution

