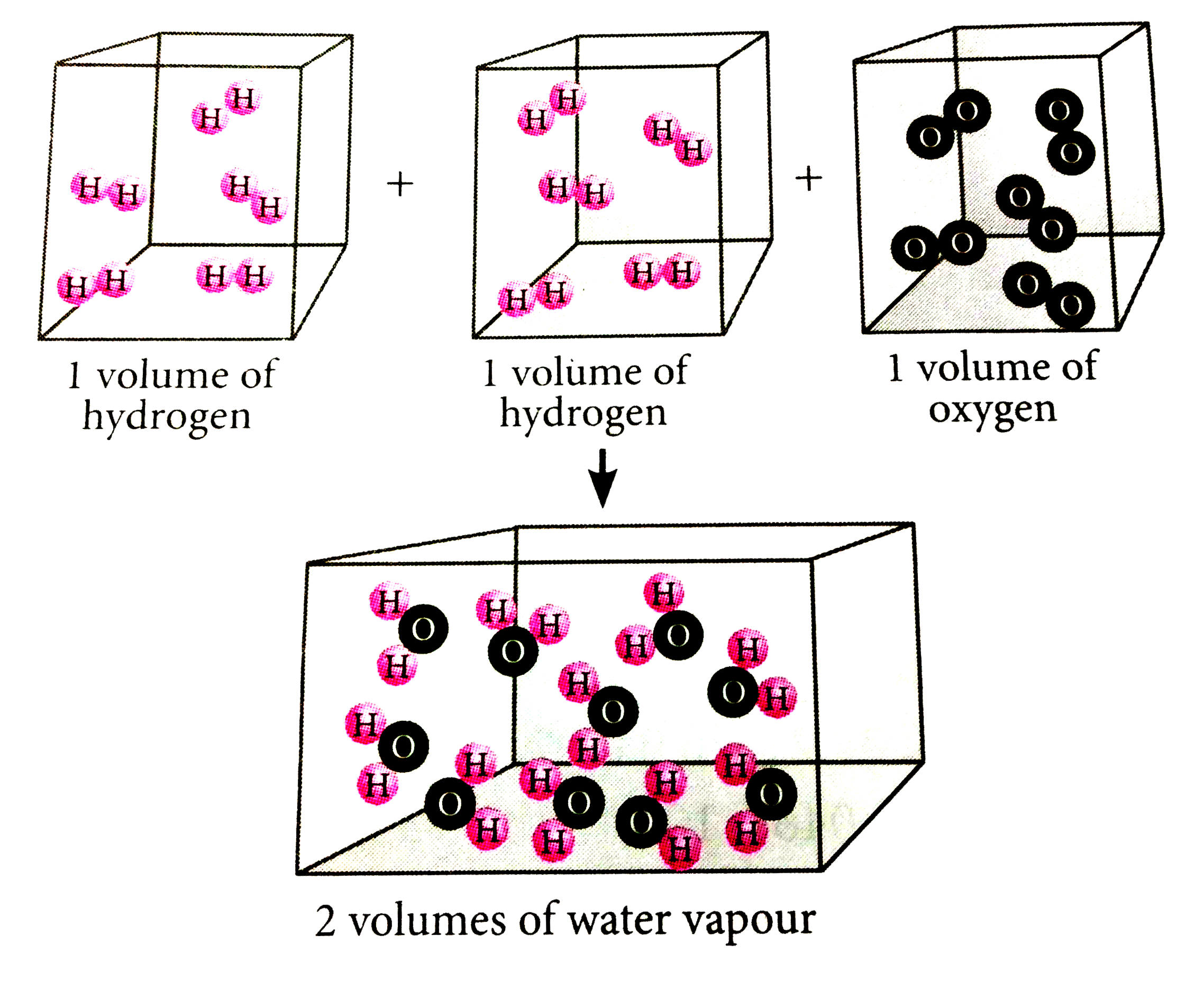
Which of the following laws of chemical combinations cannot be explained by Dalton's atomic theroy?
Law of conservation of mass
Law of constant composition
Law of multiple proportions
Law of combining volumes
The correct Answer is:D
Because Dalton's atomic theroy talks only about the masses of atoms, it does not consider the volume occupied by the atoms and molecules.
Similar Questions
Knowledge Check
Which of the following laws of chemical combination sis illustrated by the balanced chemical equation?
ALaw of multiple proportionsBLaw of definite proportionsCLaw of reciprocal proportionsDLaw of conservation of massConsider the following statement. (i) Atoms of H,O,N and C have identical properties but different mass. (ii)Matter is divisible into atoms which are further indivisible. (iii)The ratio of N:H∈NH3isl3andN:O in nitric oxide is 2:1. (iv)Dalton's atomic theory support law of conservation of mass. Which of the following pairs of statements is true according to Dalton's atomic theory?
A(i) and (ii)B(ii) and (iii)C(ii) and (iv)D(i) and (iv)Which of the following postulates of Dalton's atomic theory explains the law of constant proportion?
AAtoms of given element are identical in mass and chemical properties.BAtoms combine in the ratio of small whole numbers to form compounds.CThe relative number and kind of atoms are constant in a given compound.DAll of these
Laws OF chemical combination||Numericals
View SolutionWhat are laws of chemical combinations ? Discuss any three laws in detail.
View SolutionExplain Dalton's atomic theory of matter on the basis of law of chemical combination and other related chemical observations.
View SolutionWhich of the following is not the basic postulates of Dalton's atomic theory ?
View SolutionWhich of the following hypotheses of Dalton's atomic theorey explains the law of constant composition?
(i) All mater is composed of atoms.
(ii) Atoms cannot be created, divided or destroyed.
(iii) All the atoms of one element are alike, and different from these of any other element.
(iv) Atoms combine together in the ratio of small whole numbers.
View Solution