Hematitic (Fe2O3) is an important ore of iron. The free metal (Fe) is obtained by reducing hematite with carbon monoxide (CO) in a blast furnace:
Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g)
How many grams of Fe can be produced from 1.00kg Fe2O3 ?
A
698g
B
786g
C
896g
D
968g
Video Solution
Text Solution
The correct Answer is:A
Mass of Fe2O3→ Moles of Fe2O3→ Moles of Fe→ Mass of Fe
1.00×103gFe2O3×1molFe2O3160gFe2O3×2molFe1molFe2O3
×55.8gFe1molFe=698gFe
|
Similar Questions
Knowledge Check
What is the value of Δs∘ for the reaction below?
Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g)
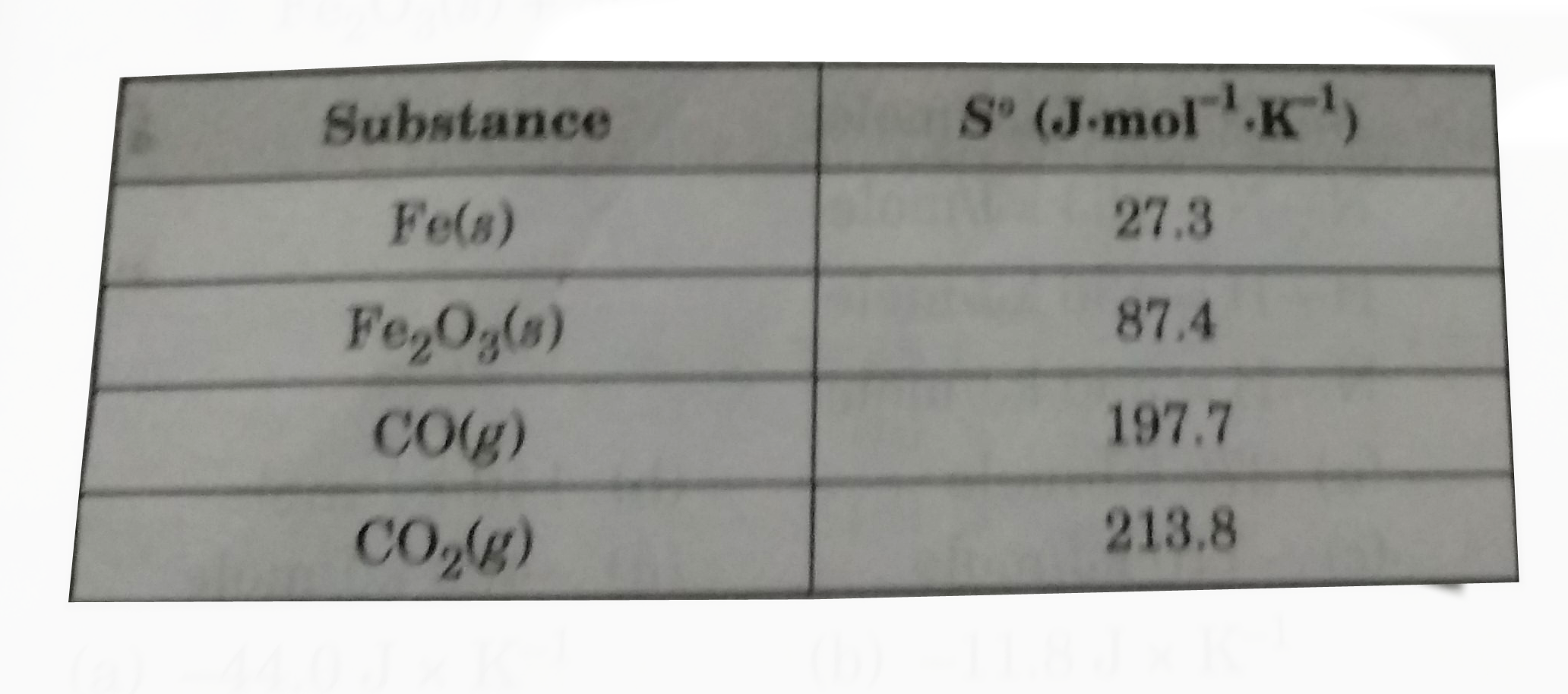 A−44.0J×K−1B−11.8J×K−1C15.5J×K−1D42.8J×K−1
A−44.0J×K−1B−11.8J×K−1C15.5J×K−1D42.8J×K−1Iron is obtained on a large scale from Fe2O3 by
AReduction with AlBReduction with COCReduction with H2DReduction with sodiumFe2O3(S)+2AI(s)→2Fe(l)+AI2O3(s) + Heat . This reaction is called .
AcalcinationBthermit reactionCthermal reactionDdisplacement .
Identify oxidizing agent in the following reactions:
Fe2O3+3CO→2Fe+3CO2
View SolutionMoles of iron which can be made from Fe2O3 by the use of 294 g of carbon monoxide in the reaction :
Fe2O3+3CO→2Fe+CO2 are:
View SolutionHow many moles of iron can be made from Fe2O3 by the use of 16 mol of carbon monoxide in the following reaction :
Fe2O3+3CO→2Fe+3CO2
View SolutionHow much iron can be theoretically obatined by the reduction of 1.0kg of Fe2O3?(At.wt.of Fe=56)
View SolutionMagnetite, Fe3O4, can be converted into metallic iron by heating with carbon monoxide as represented by this equation:
Fe3O4(s)+CO(g)→Fe(s)+CO2(g)
The kilograms of Fe3O4 which must be processed in this way to obtain 5.00kg of iron, if the process is 85% efficient is closest to? [M:=Fe=56]
View Solution
