How many grams of oxygen (O2) gas reacts completely with 1.0g of calcium?
A
0.6g
B
1.6g
C
4.0g
D
0.4g
Video Solution
Text Solution
The correct Answer is:D
Ca+O2→CaO
According to formula,
Moles of C = Moles of O
MassCaMolar massCa=MassOMolar MassO
1gCa40gCa=mO16gO
∴MO=0.4g
We can use this approach because the enite Ca is changed into CaO.
|
Similar Questions
Knowledge Check
How many grams of H3PO4 is required to completely neutralize 120 g of NaOH
A49B98C196D9.8How many milli grams of Fe0.9O reacts completely with 10 mL 0.1 M KMnO4 solution in acidic conditions ? (Fe =56)
A47B402C534D57027g of Al will react completely with…… g of O2
A8gB10gC24gD49g
How many grams of chlorine are required to completely react with 0.40 g of hydrogen (HJ to yield hydrochloric acid (HCl) ? Also calculate the amount of HCl formed.
View SolutionHow many moles are in 96gO2?
View SolutionHow many moles of acetic anhydride (Ac2O) is required to react completely with 1 mole of sucrose ?
View SolutionHow many grams of oxygen are liberated on decomposition of 12.26 g of KClO3?
View SolutionHow many moles of acetic anhydride (Ac2O) is needed to react completely with tataric acid, ribose and glucose respectively,
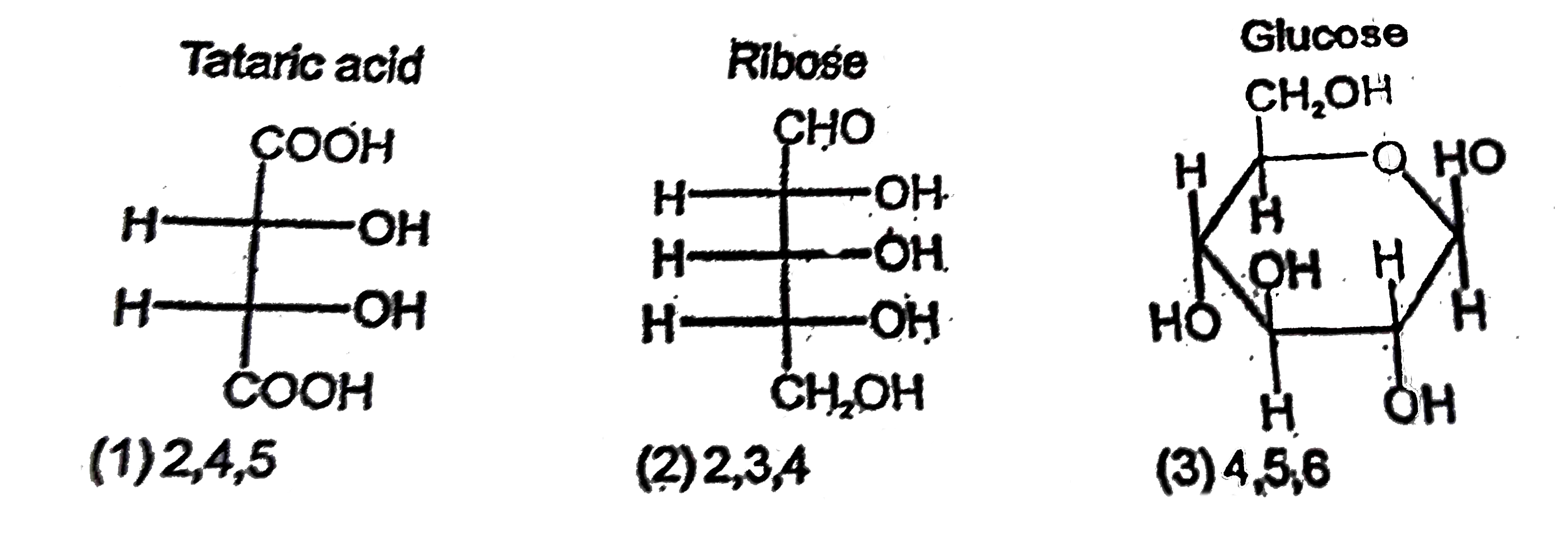
View Solution

