The electronegativity of the following elements increases in the order
P,Si,N,C
Si,P,C,N
C,N,Si,P
N,Si,C,P
The correct Answer is:B
In general, electronegativity increases across the period on account of increase of Zeff while it decreases on moving down the group due to increase of atomic size:
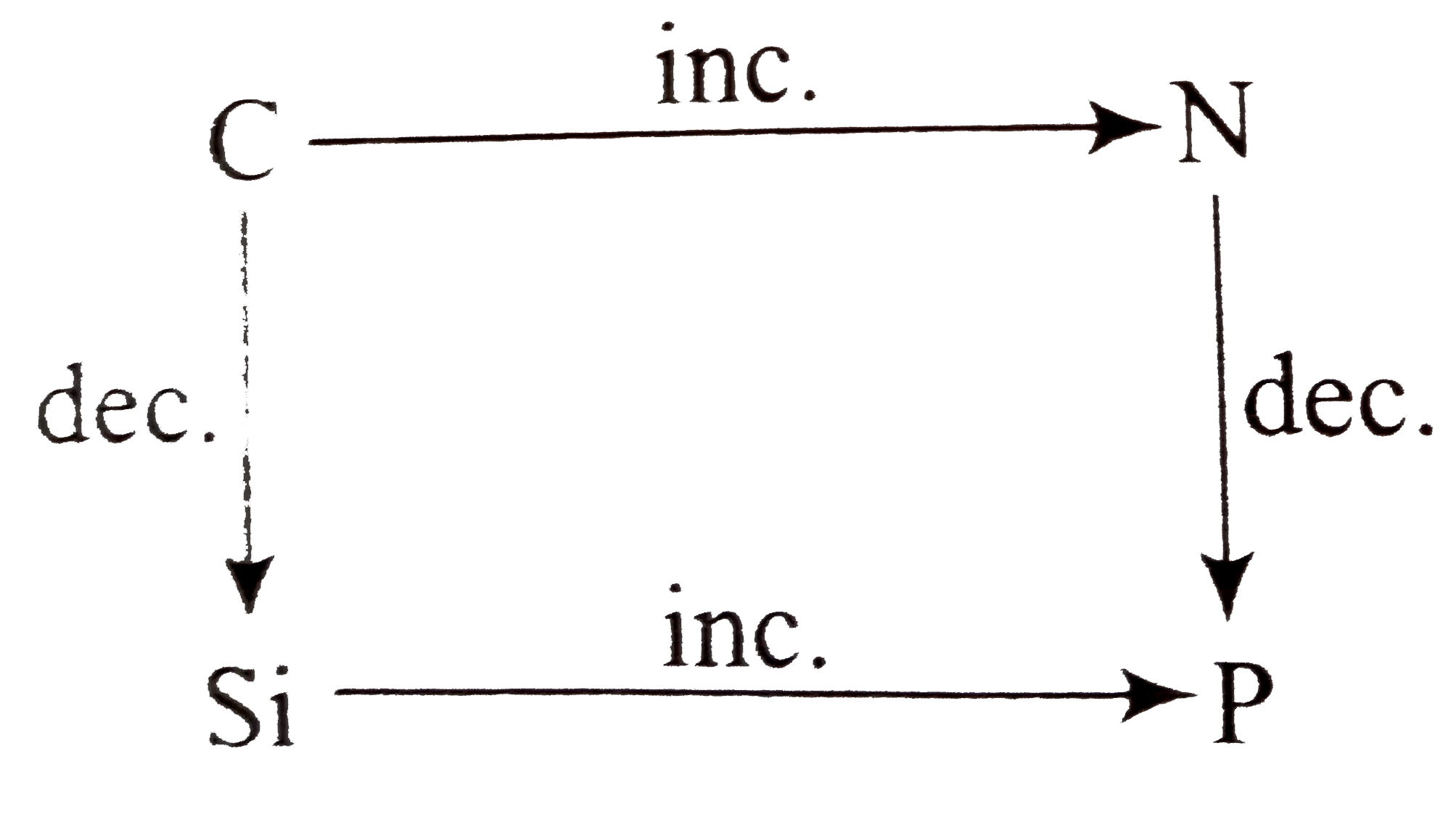
Thus, the correct order is
Si(1.8)>P(2.1)>C(2.5)>N(3.0)
Similar Questions
The EN of the elements C,N,Si and P increases in the order of ______ .
View SolutionMetals have few electrons in their valence shell while non-metals generally have more electrons in their valence shell. Metallic character is closely related to atomic radius and ionisation enthalpy. Metallic character increases from top to bottom in a group and decreases from let to right in a period of periodic table. metallic character is inversely related to electronegativity of element.
Q. The electronegativity of the following elements increase in the order:
View Solution
Knowledge Check
Metals have few electrons in their valence shell while non-metals generally have more electrons in their valence shell. Metallic character is closely related to atomic radius and ionisation enthalpy. Metallic character increases from top to bottom in a group and decreases from let to right in a period of periodic table. metallic character is inversely related to electronegativity of element.
Q. The electronegativity of the following elements increase in the order:AC, N, Si, PBN, Si, C, PCSi, P, C, NDP, SI, N, CThe electronnegativity of the following elements increases in the order
AC,N,Si,PBN,Si,C,PCSi,P,C,NDP,Si,N,CThe electronegativity of the following elements increases in the order
AC, N, Si, PBN, Si, C, PCSi, P, C, NDP, Si, N, C
Electronegativity of the elements O,F,S and Cl increases in the order
View SolutionThe correct order of increasing electronegativity of the following elements is:
View SolutionWhich is true about the electronegativity order of the following elements ?
View SolutionThe electronegativity follows the order:
View SolutionAssertion : Acidity of hydrides of group 16 elements increases down the group.
Reason : Electronegativity of these elements increases down the group.
View Solution
