Which of the following conditions are fulfilled by the Lewis dot structure for carbon tetrachloride?
(i) Each covalent bond is formed as a result of sharing of an electron pair between the atoms.
(ii) Each combining atom contributes at least one electron to the shared pair.
(iii) The combining atoms attain the noble gas configurations as a result of the sharing of electrons.
A
(i), (ii)
B
(ii), (iii)
C
(i), (iii)
D
(i), (ii), (iii)
Video Solution
Text Solution
The correct Answer is:D
All the conditions are fullfilled by the Lewis dot structure for Cl4:
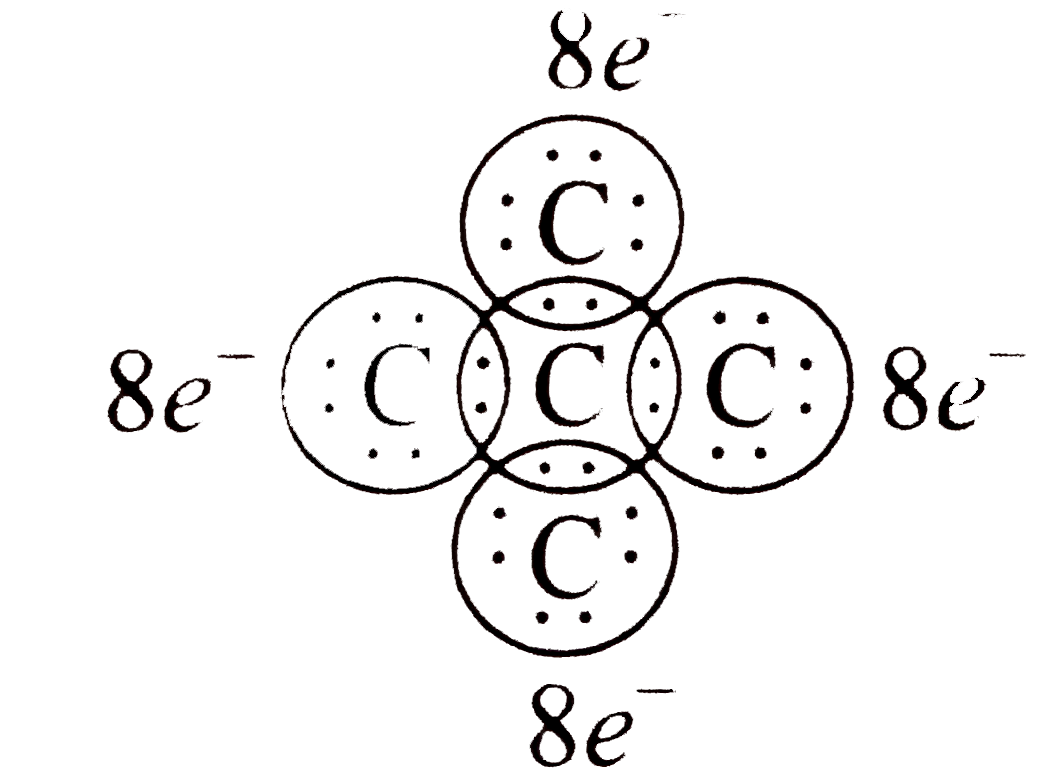
|
Similar Questions
Knowledge Check
A covalent bond results from the sharing of electrons between two atoms of
Ametallic elementsBnonmetallic elementsCmetalloid elementsDzero or relatively small electronegativity differenceDuring the formation of CCl4 molecule, each chlorine atom contributes _________ electron(s) to the shared pair.
AoneBtwoCthreeDfourA bond formed by sharing of electrons between atoms of the elements is called
Aionic bondsBcovalent bondsCco-ordinate bondDnone
The compounds formed by mutual sharing of electrons between the atoms are called covalent compounds.
View SolutionSharing of three electron pairs between two atoms results in the formation of a ______ bond.
View SolutionThe bond formed by sharing of electron pair is contributed by one of the two bonded atoms is known as
View SolutionWhat type of bond is formed when two atoms combine by sharing of electrons ?
View SolutionThe unequal sharing of bonded pair of electrons between two atoms in a molecule causes ___________.
View Solution

