Which of the following is the structure of N2O which is isoelectronic with CO2 and N−3?
A
N−N−O
B
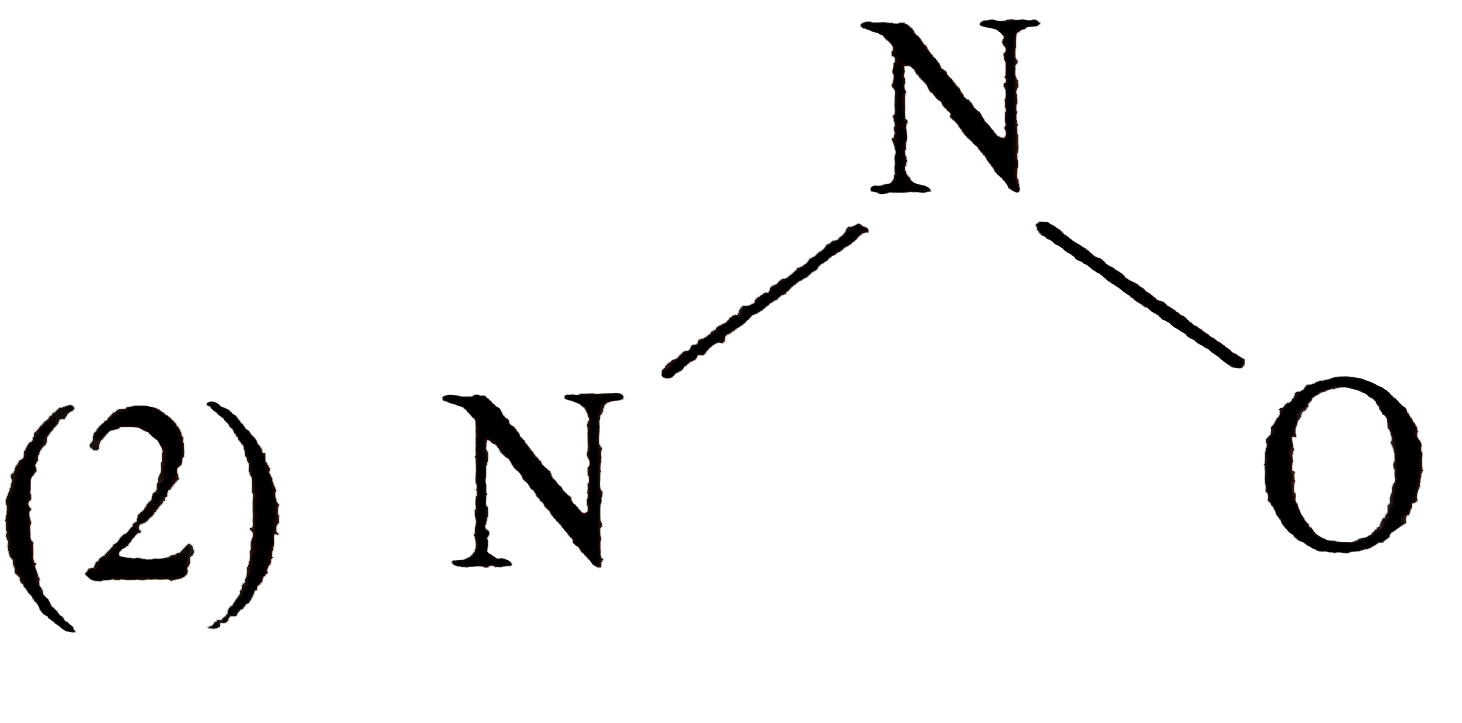
C
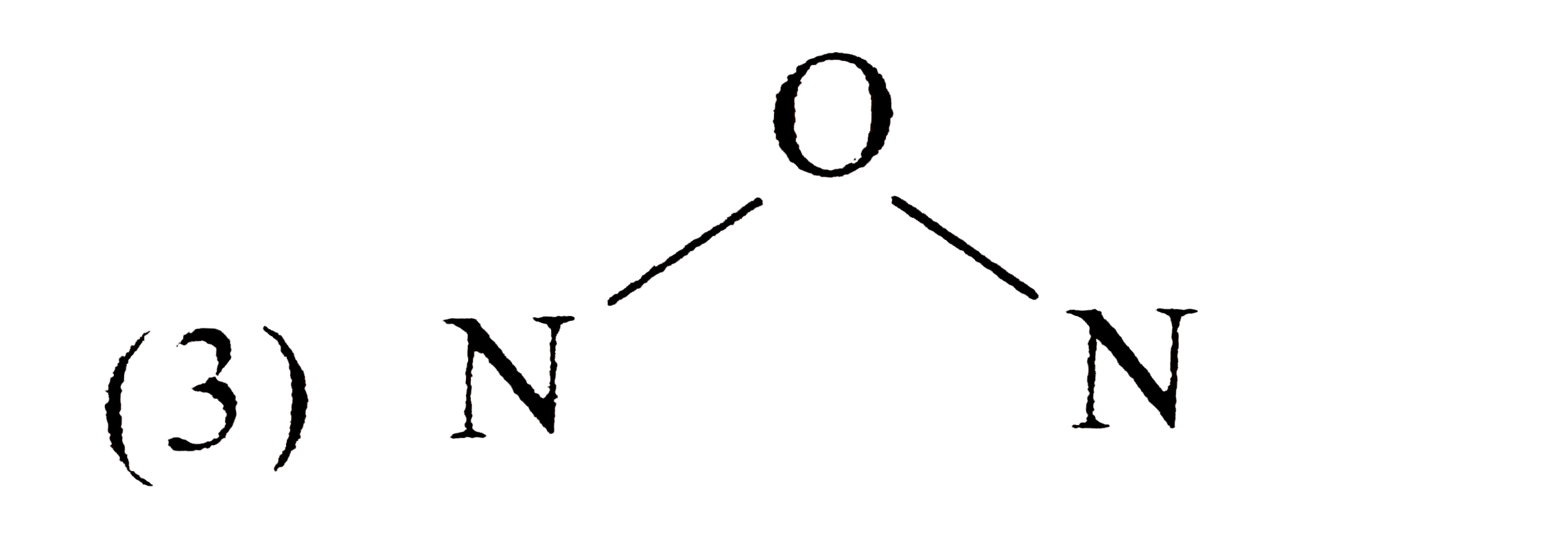
D
N−O−N
Video Solution
Text Solution
The correct Answer is:A
According to isoelectronic principle, isoelectronic species usually have the same geometry. Thus, like CO2 and N−3, N2O is also linear. It is actually a resonance hybrid
..N..=N=..O..↔:N≡N−..O..:
|
Similar Questions
Knowledge Check
Which of the following is not correct for N2O?
AIt is called laughing gasBIt is nitrous oxideCIt is not a linear moleculeDIt is least reactive in all oxides of nitrogenN2 is isoelectronic with :
ACOBNO+CC2H2DCH4Which of the following is not correct for N2O?
AIt is called laughing gasBIt is nitrous oxideCIt is not a linear moleculeDIt is least reactive in all oxides of nitrogen

