How many types of F−S−F bonds are present in SF4?
A
5
B
4
C
2
D
3
Video Solution
Text Solution
The correct Answer is:C
SF4 has trigonal bipyramidal geometry but seesaw shape due to a lone pair.
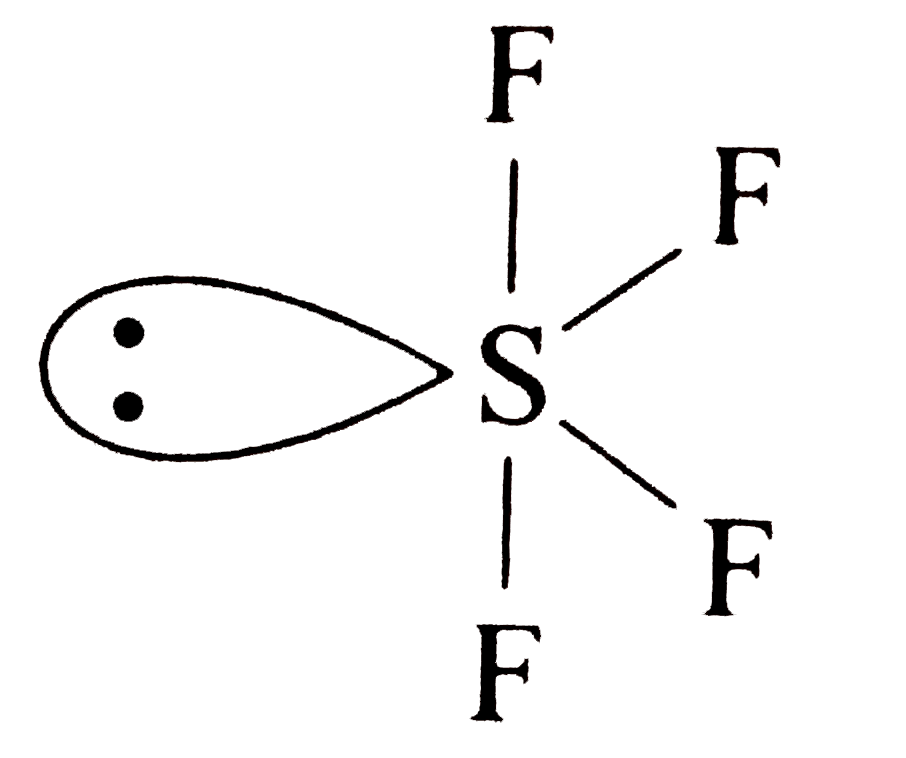
It has two types of bonds: axial bonds (164±) and equatorial bonds (155±). Note that axial bonds are longer than equatorial bonds.
|
Similar Questions
Knowledge Check
How many and what types of bonds are present in NH+4?
AFour covalent bondsBThree covalent bonds and one ionic bondCFour ionic bondsDThree covalent bonds and one coordinate bondHow many types of tissues are present in stomach
AOral mucosaBGastric mucosaCThree types onlyDAll four typesHow many π - bonds are present in naphthalene ?
A4B5C6D7
