The correct order of bond order values among the following
(i) NO− (ii) NO+
(iii) NO (iv) NO2+
(v) NO2−
(i) lt (iv) lt (iii) lt (ii) lt (v)
(iv) = (ii) lt (i) lt (v) lt (iii)
(v) lt (i) lt (iv) = (iii) lt (ii)
(ii) lt (iii) lt (iv) lt (i) lt (v)
The correct Answer is:C
To determine the correct order of bond order values among the species NO−, NO+, NO, NO2+, and NO2−, we will calculate the bond order for each species using the molecular orbital theory. The bond order is calculated using the formula:
Bond Order=Nb−Na2
where Nb is the number of electrons in bonding molecular orbitals and Na is the number of electrons in anti-bonding molecular orbitals.
Step 1: Calculate the bond order for NO
1. Count the total number of electrons:
- Nitrogen (N) has 7 electrons and Oxygen (O) has 8 electrons.
- Total = 7+8=15 electrons.
2. Molecular orbital configuration:
- The configuration is:
σ21sσ∗21sσ22sσ∗22sσ22pzπ22pxπ22pyπ∗12pxπ∗02py
- Bonding electrons Nb=10 (from σ21s,σ22s,σ22pz,π22px,π22py)
- Anti-bonding electrons Na=5 (from π∗12px,π∗02py)
3. Calculate bond order:
Bond Order=10−52=2.5
Step 2: Calculate the bond order for NO−
1. Total electrons:
- 15+1=16 electrons.
2. Molecular orbital configuration:
- The configuration is:
σ21sσ∗21sσ22sσ∗22sσ22pzπ22pxπ22pyπ∗12pxπ∗12py
- Bonding electrons Nb=10
- Anti-bonding electrons Na=6
3. Calculate bond order:
Bond Order=10−62=2
Step 3: Calculate the bond order for NO+
1. Total electrons:
- 15−1=14 electrons.
2. Molecular orbital configuration:
- The configuration is:
σ21sσ∗21sσ22sσ∗22sσ22pzπ22pxπ22pyπ∗02pxπ∗12py
- Bonding electrons Nb=10
- Anti-bonding electrons Na=4
3. Calculate bond order:
Bond Order=10−42=3
Step 4: Calculate the bond order for NO2+
1. Total electrons:
- 15−2=13 electrons.
2. Molecular orbital configuration:
- The configuration is:
σ21sσ∗21sσ22sσ∗22sσ22pzπ22pxπ12pyπ∗02pxπ∗02py
- Bonding electrons Nb=9
- Anti-bonding electrons Na=4
3. Calculate bond order:
Bond Order=9−42=2.5
Step 5: Calculate the bond order for NO2−
1. Total electrons:
- 15+2=17 electrons.
2. Molecular orbital configuration:
- The configuration is:
σ21sσ∗21sσ22sσ∗22sσ22pzπ22pxπ22pyπ∗12pxπ∗12py
- Bonding electrons Nb=10
- Anti-bonding electrons Na=7
3. Calculate bond order:
Bond Order=10−72=1.5
Summary of Bond Orders:
- NO+: 3
- NO: 2.5
- NO2+: 2.5
- NO−: 2
- NO2−: 1.5
Final Order:
The correct order of bond order values is:
1. NO+ (3)
2. NO = NO2+ (2.5)
3. NO− (2)
4. NO2− (1.5)
- Nitrogen (N) has 7 electrons and Oxygen (O) has 8 electrons.
- Total = 7+8=15 electrons.
- The configuration is:
σ21sσ∗21sσ22sσ∗22sσ22pzπ22pxπ22pyπ∗12pxπ∗02py
- Bonding electrons Nb=10 (from σ21s,σ22s,σ22pz,π22px,π22py)
- Anti-bonding electrons Na=5 (from π∗12px,π∗02py)
Bond Order=10−52=2.5
- 15+1=16 electrons.
- The configuration is:
σ21sσ∗21sσ22sσ∗22sσ22pzπ22pxπ22pyπ∗12pxπ∗12py
- Bonding electrons Nb=10
- Anti-bonding electrons Na=6
Bond Order=10−62=2
- 15−1=14 electrons.
- The configuration is:
σ21sσ∗21sσ22sσ∗22sσ22pzπ22pxπ22pyπ∗02pxπ∗12py
- Bonding electrons Nb=10
- Anti-bonding electrons Na=4
Bond Order=10−42=3
- 15−2=13 electrons.
- The configuration is:
σ21sσ∗21sσ22sσ∗22sσ22pzπ22pxπ12pyπ∗02pxπ∗02py
- Bonding electrons Nb=9
- Anti-bonding electrons Na=4
Bond Order=9−42=2.5
- 15+2=17 electrons.
- The configuration is:
σ21sσ∗21sσ22sσ∗22sσ22pzπ22pxπ22pyπ∗12pxπ∗12py
- Bonding electrons Nb=10
- Anti-bonding electrons Na=7
Bond Order=10−72=1.5
- NO+: 3
- NO: 2.5
- NO2+: 2.5
- NO−: 2
- NO2−: 1.5
The correct order of bond order values is:
1. NO+ (3)
2. NO = NO2+ (2.5)
3. NO− (2)
4. NO2− (1.5)
Similar Questions
Knowledge Check
The correct order of bond order values among the following
(i) NO− (ii) NO+
(iii) NO (iv) NO2+
(v) NO2−AA<D<C<B<EBD=B<A<E≤CCE<A<D=C<BDB<C<D<A<EThe correct order of C-O bond length among
ACO<CO2<CO2−3BCO2<CO2−3<COCCO<CO2−3<CO2DCO2−3<CO2<COThe correct bond order among the following species is :
AN2−2>N−2>N2>N+2BN2>N−2=N+2>N2−2CN2−2>N+2>N−2>N2DN−2>N2=N2−2>N+2
The correct order of I.E2 among the following
View SolutionThe decreasing order of acidic character of the following is:
(I)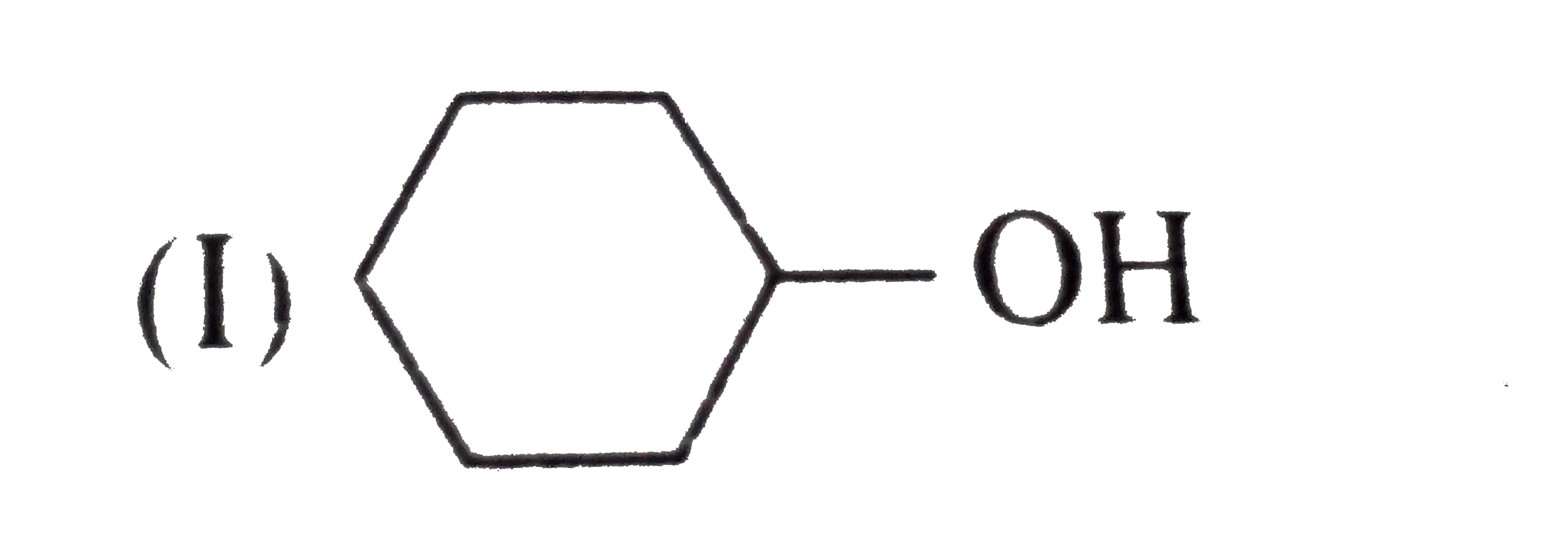 (II)
(II) 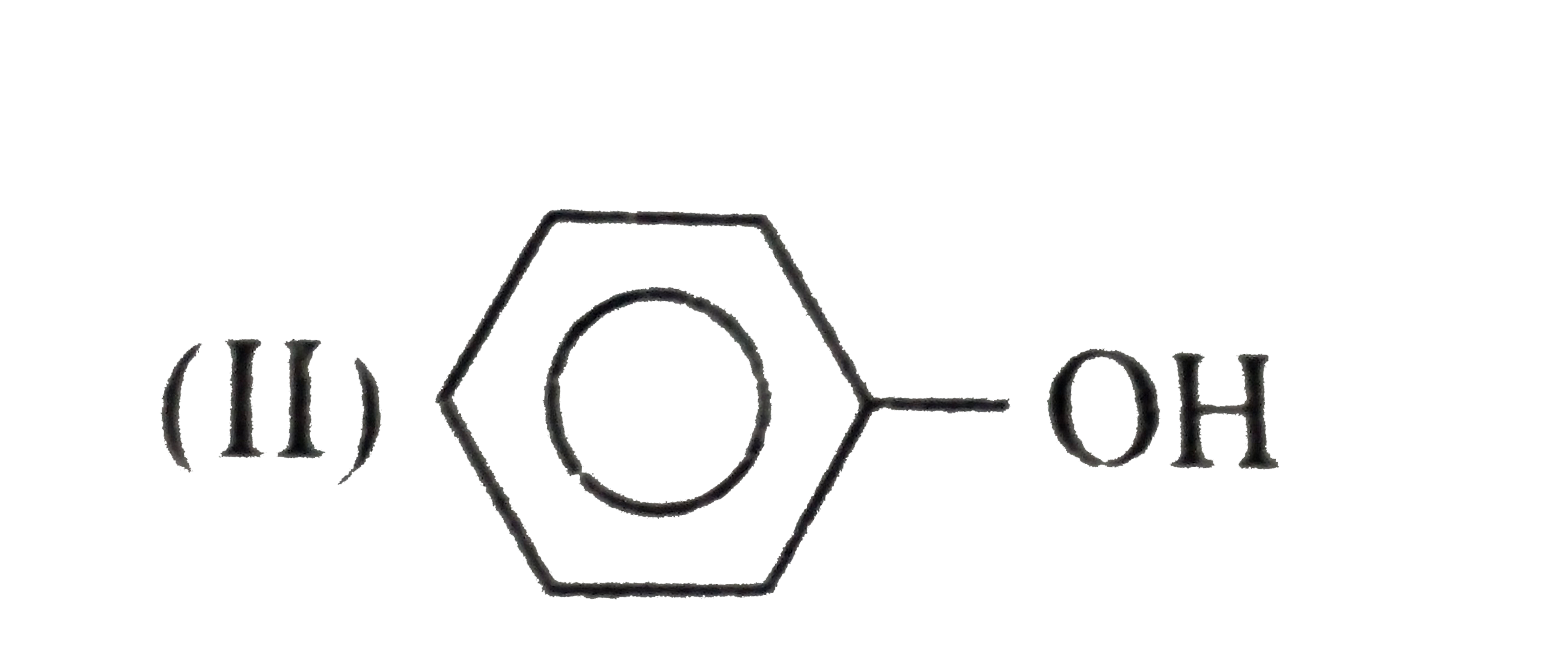
(III)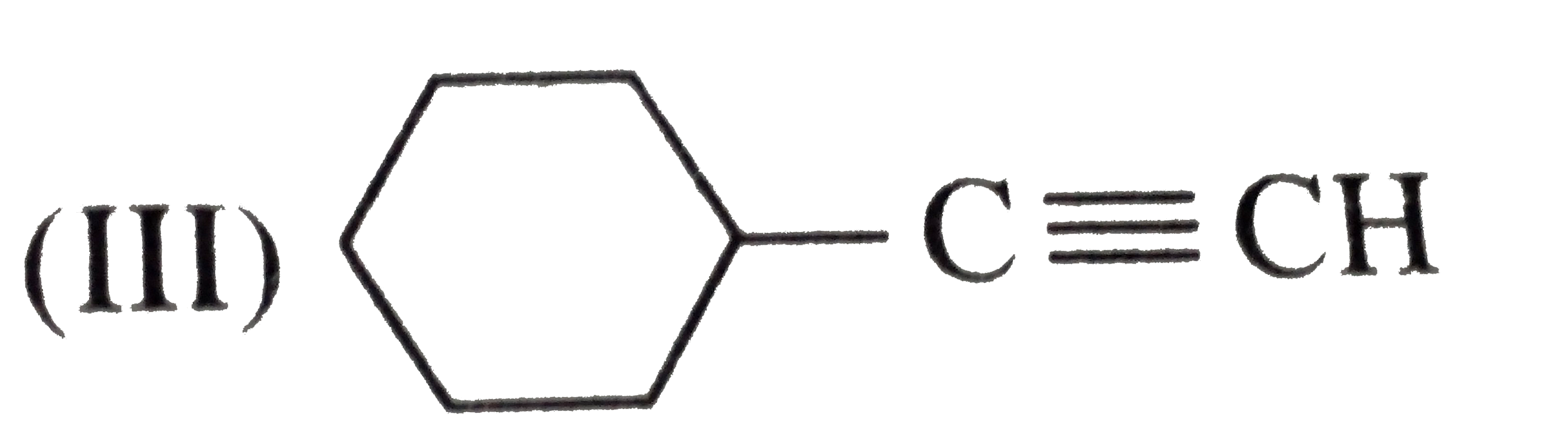 (IV)
(IV) 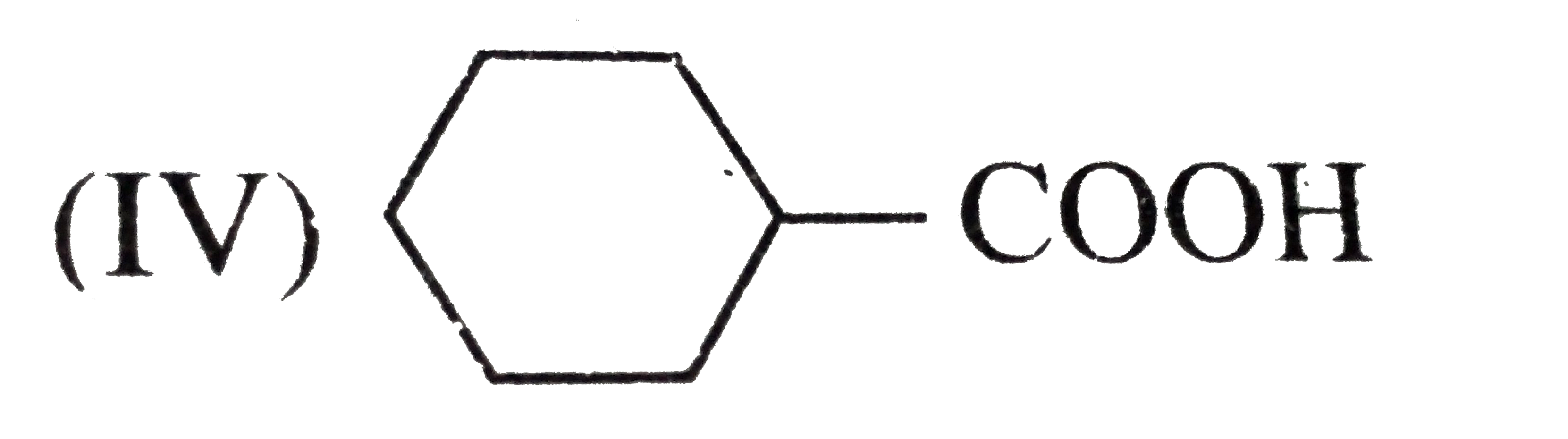
View SolutionThe correct order of electron affinity among
(i) Na (ii) Mg (iii) F (iv) I is
View SolutionThe correct order of decreasing C−O bond length of (1) CO,(II)CO2−3(III)CO2 is .
View SolutionWhat is the order of polarity of the following bond?
(i) C-H (ii) F-H (iii) Br-H (iv) Na-I (v) K-F (vi) Li-Cl
View Solution
