The slope of the plot between pV and p at constant temperature is
A
negative
B
positive
C
1/2
D
zero
Video Solution
Text Solution
The correct Answer is:D
According to Boyle's law , we have
pV=constant(n,Tconstant)
The value of the constant depends on the number of moles (n) and temperature (T) . Thus , the plot of pV against p at constant temperature is a horizontal line:
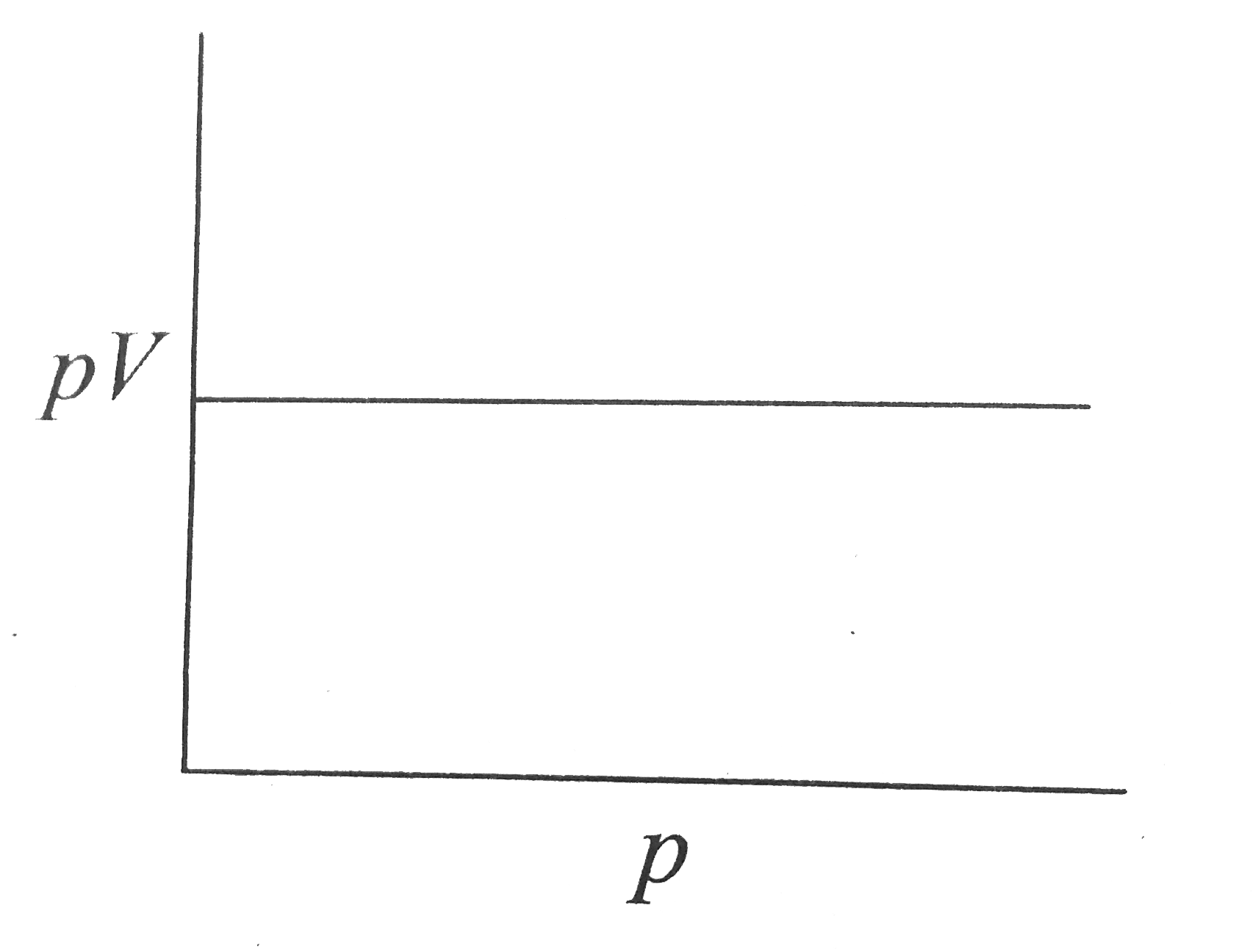
Thus, the slope is zero.
|
Similar Questions
Knowledge Check
Slop between pV and p at constant temperature is
Aslope between pV and p is zeroBslope between pV and p is 1Cslope between pV and p is 12Dslope between pV and p is 1√2Slope of the plot between V and P at constant temperature
AzeroB1C1//2D1√2Graph between p and V at constant temperature is
AstraightBcurved increasingCstraight line with slopeDnone of these
What is the nature of curve by plotting P against PV at constant temperature for a gas ? What is the name assigned to this curve ?
View SolutionThe graph between PV vs P at constant temperature is linear parallel to the pressure axis.
View SolutionGraph between p and V at constant temperature is
View SolutionThe slopes between pV versus V and pV versus p at constant temperature ae respectively
View SolutionWhich one of the following plots will be a parabola at constant temperature?
View Solution

