Graph between p and V at constant temperature is
A
straight
B
curved increasing
C
straight line with slope
D
none of these
Video Solution
Text Solution
The correct Answer is:D
Since p∝1/V(constant T,n) according to Boyle's law , the graph is a rectangular hyperbola:
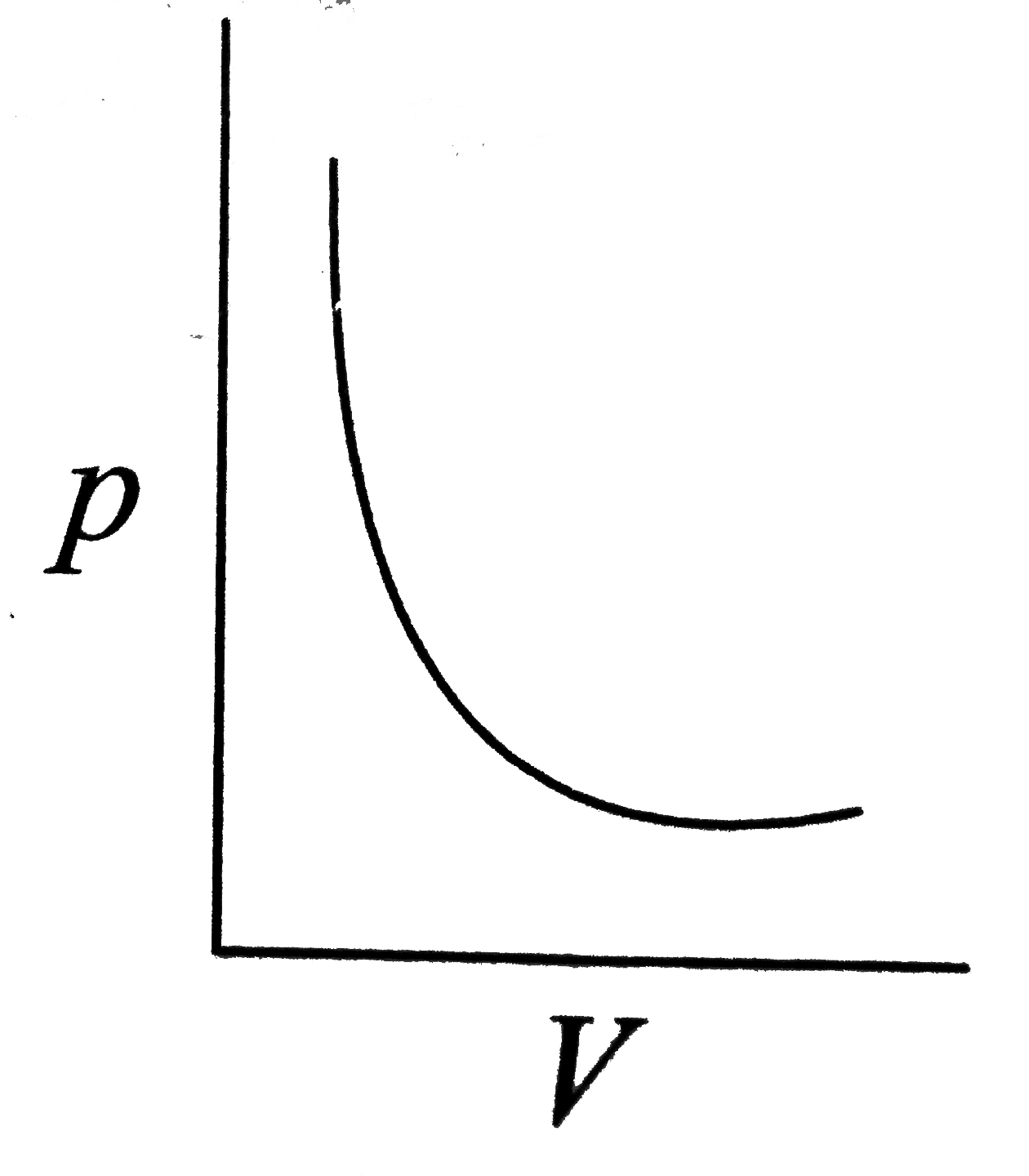
|
Similar Questions
Under constant temperature, graph between P and 1/V is a :
View SolutionUnder constant temperature, graph between P and 1/V is a :
View SolutionThe slope of the graph between log P and log V at constant temperature for a given mass of gas is
View SolutionSlope of the plot between V and P at constant temperature
View Solutionनियत ताप पर Pऔर Vके बीच का ग्राफ है
View SolutionUnder constant temperature, graph between P and 1/V is a
View SolutionDraw a graph between pressure (P) vs volume (1V) at constant temperature.
View SolutionFor a certain mas of the gas at constant temperature draw a graph between the volume against the corresponding pressure i.e. V vs P.
View Solution
