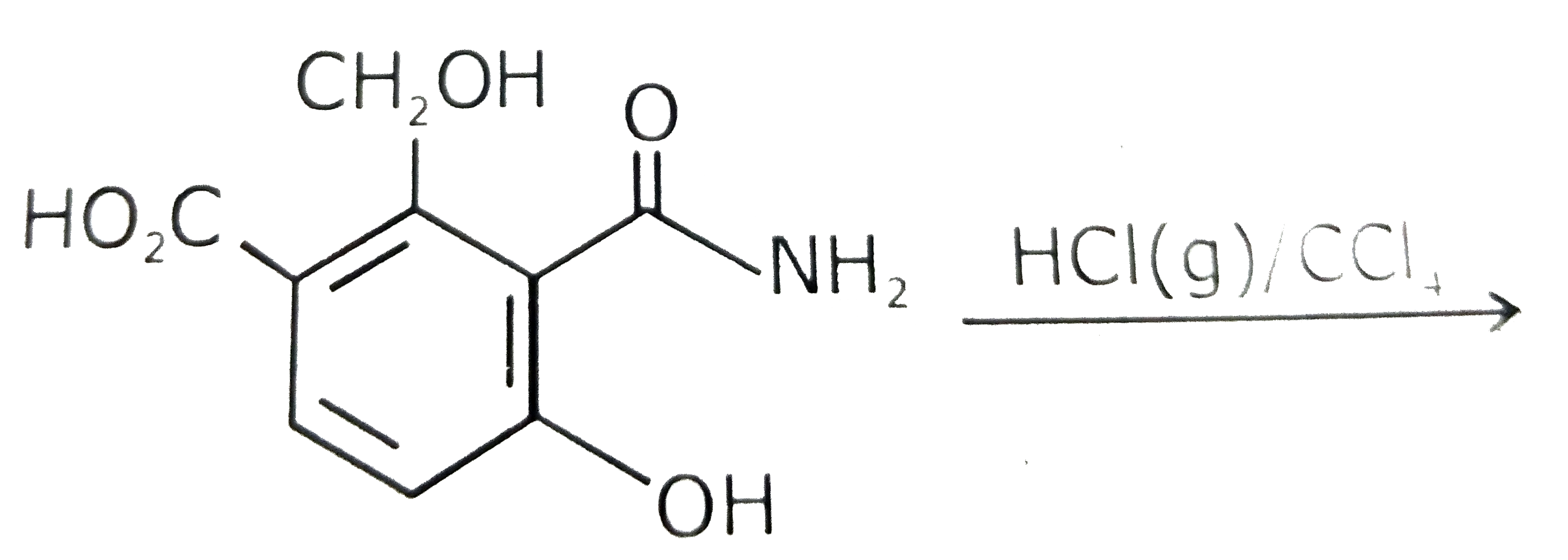
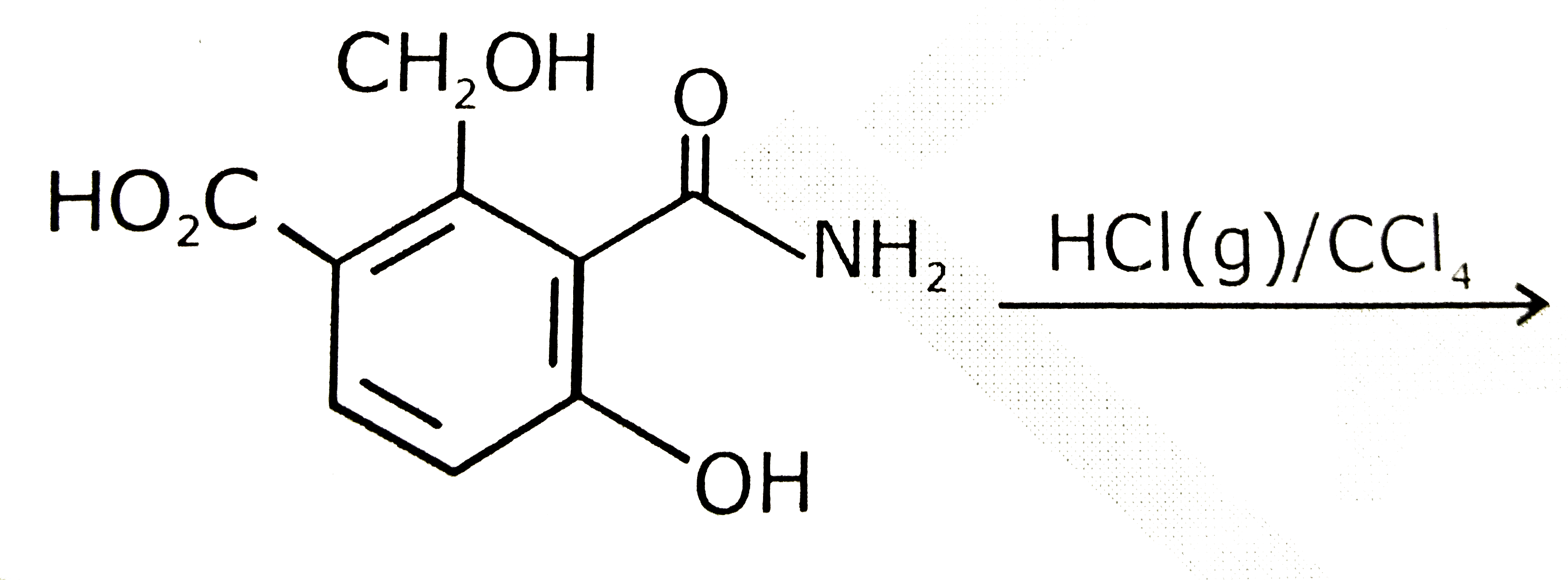What products are expected from the desproprtionation reactin of hypochorous acid ?
A
HClO2 and HClO4
B
HClO3 and Cl2O
C
HCl and Cl2O
D
HCl and HClO3
Video Solution
Text Solution
The correct Answer is:D
The hypohalite ions tend to disproprtionate, particularly in basic solution . The rate of desproportionation reaction increases with temperature:
3OX−→2X−+XO−3
or 3HO+1CI→2H−1CI+H+5CIO3.
|
Topper's Solved these Questions
- REDOX REACTIONSBook:R SHARMAChapter:REDOX REACTIONSExercise:Archives
Explore 29 Videos
- REDOX REACTIONSBook:R SHARMAChapter:REDOX REACTIONSExercise:Follow-up Test 7
Explore 10 Videos
- PURIFICATION AND CHARATERIZATION OF ORGANIC COMPOUNDSBook:R SHARMAChapter:PURIFICATION AND CHARATERIZATION OF ORGANIC COMPOUNDSExercise:Archives
Explore 38 Videos
- SOME BASIC CONCEPTS OF CHEMISTRYBook:R SHARMAChapter:SOME BASIC CONCEPTS OF CHEMISTRYExercise:Archives
Explore 26 Videos
Similar Questions
Knowledge Check
What products are expected from the desproprtionation reactin of hypochorous acid ?
AHCl and Cl2OBHCl and HCIO3CHCIO3 and Cl2ODHCIO2 and HCIO4Which products are expected from the disproportionation reaction of hypochlorous acid?
AHCIO3andCI2OBHCIO2andHCIO4CHCIandCI2ODHCIandHCIO3What products are expected from the disproportionation reaction of chlorine with hot concentrated solution of sodium hydroxide ?
ANaCl,NaClO3BNaCl,NaClOCNaCl,NaClO3DNaCl,NaClO2
What product (s) are expected from the following reaction ?
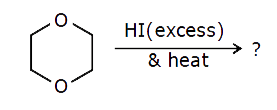
View SolutionThe major product expected from the following reaction is:
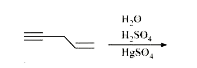
View SolutionWhat is the major product expected from the following reaction?
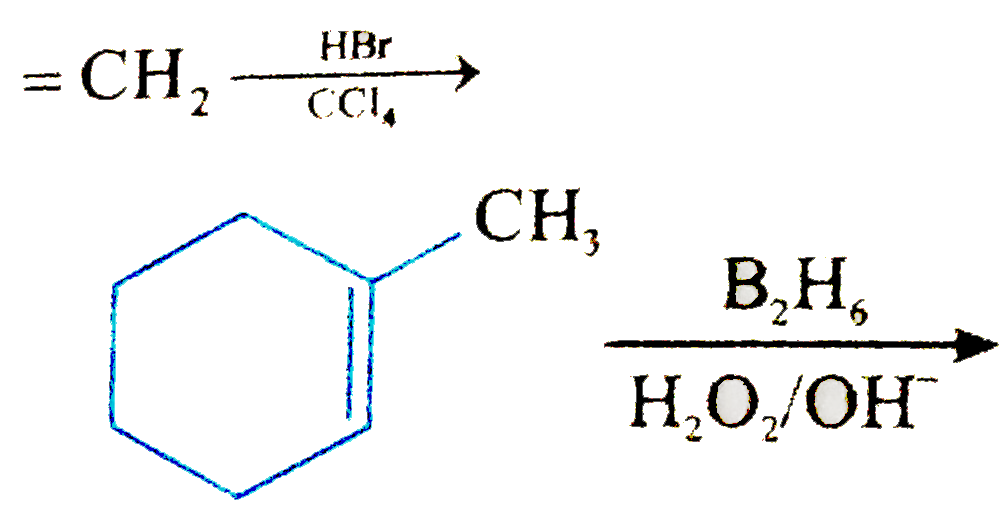
View SolutionWhat is the major product expected from the following reaction?
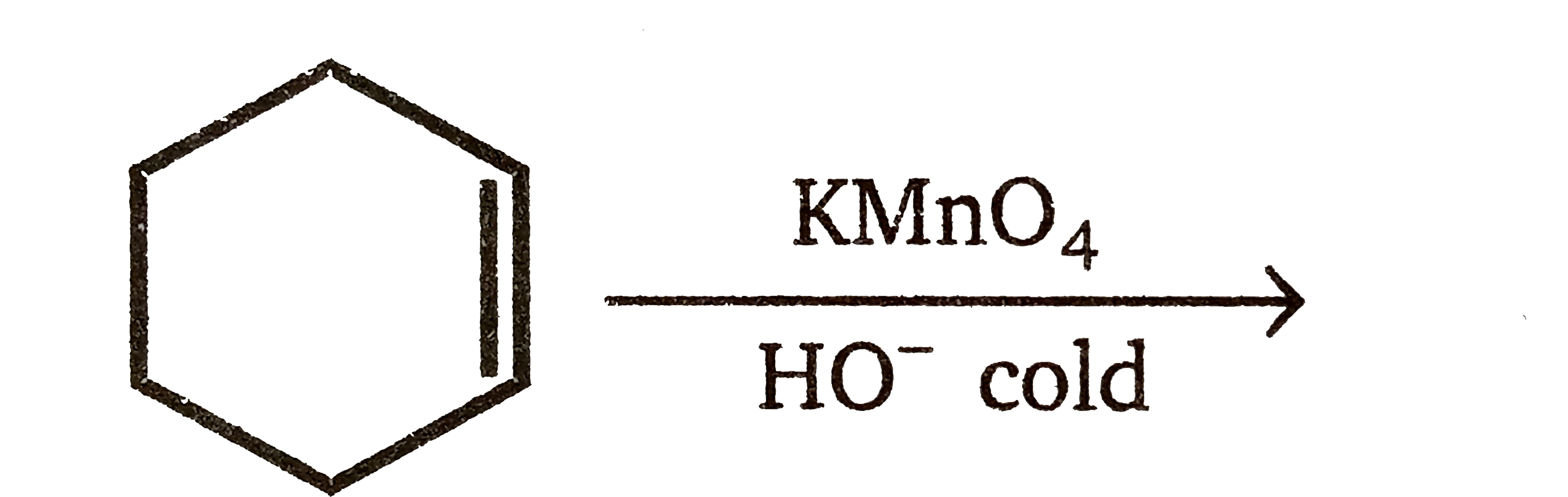
View SolutionWhat is the major product expected from the following reaction?
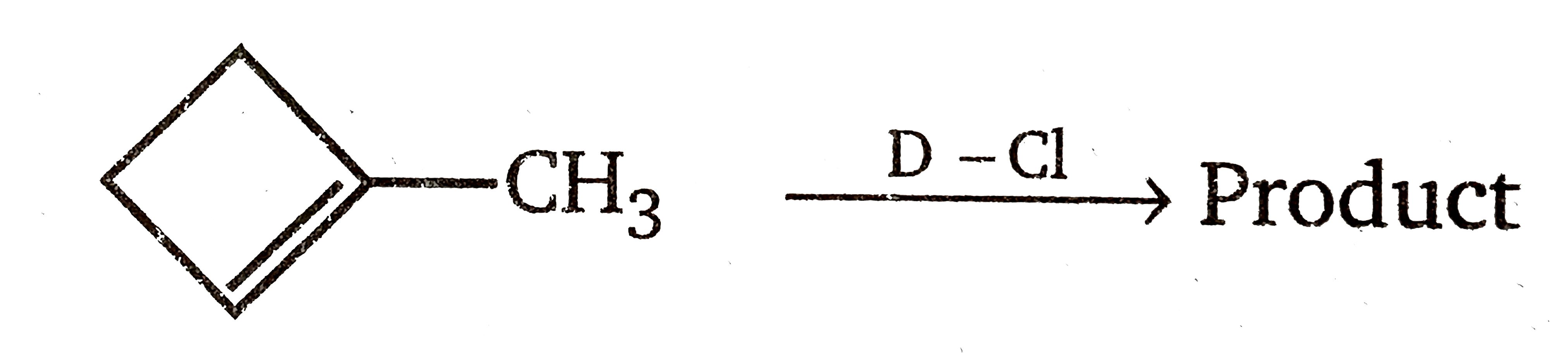
View Solution