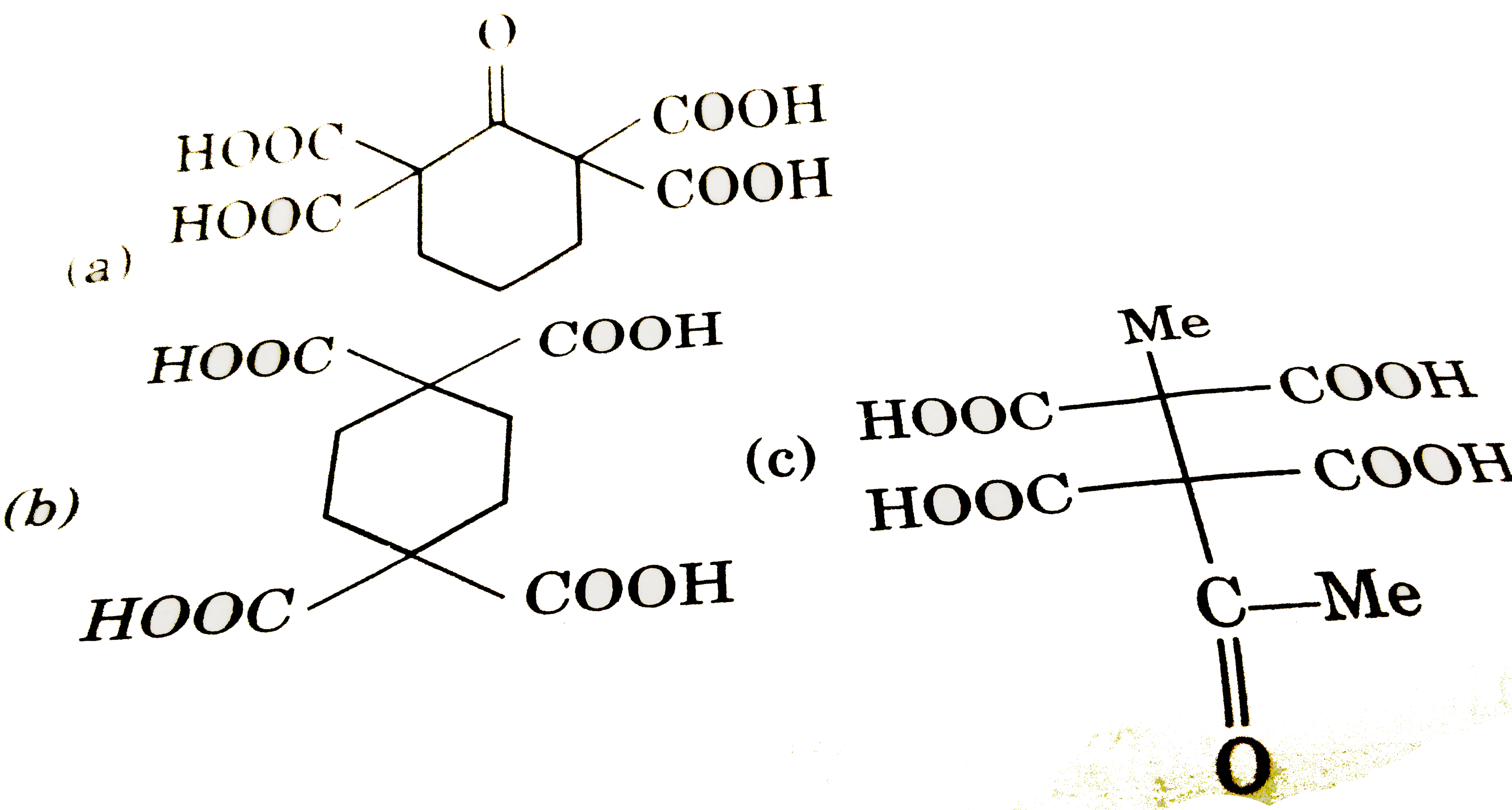
In CO2 molecule,
A
C is sp-hybridized while O's are sp2-hybridized
B
C is sp2-hybridized whike O's are sp-hybridized
C
both C's and O's are sp-hybridized
D
both C's and O's are sp2-hybridized
Video Solution
Text Solution
The correct Answer is:A
In CO2 molecule, C is sp-hybridized while O's are sp2-hybridized. Two sp-hybridized orbitals of C atom overlap with sp2-hybridized orbitals of O atoms to make two sigma bonds while unhybridized p atomic orbitals of C atom are involved in pπ−pπ multiple bonding with p atomic orbitals of oxygen atoms. As a result, unlike the dioxides of the other group 14 elements, CO2 exists in the form of discrete linear molecules:
:..O=C=..O:
|
Topper's Solved these Questions
- THE P BLOCK ELEMENTSBOOK - R SHARMACHAPTER - THE P BLOCK ELEMENTSEXERCISE - follow up test 10
8 Videos
- THE P BLOCK ELEMENTSBOOK - R SHARMACHAPTER - THE P BLOCK ELEMENTSEXERCISE - Question bank
26 Videos
- THE P BLOCK ELEMENTSBOOK - R SHARMACHAPTER - THE P BLOCK ELEMENTSEXERCISE - follow up test 8
17 Videos
- STRUCTURE OF ATOMBOOK - R SHARMACHAPTER - STRUCTURE OF ATOMEXERCISE - ARCHIVES
55 Videos
- THE S BLOCK ELEMENTSBOOK - R SHARMACHAPTER - THE S BLOCK ELEMENTSEXERCISE - ARCHIVES
27 Videos
Similar Questions
Knowledge Check
For assimilation of one CO2 molecules, the energy required in form of ATP & NADPH2
A2ATP&2NADPH2B5ATP&3NADPH2C3ATP&2NADPH2D18ATP&12NADPH2Fixation of one CO2 molecule through Calvin cycle requires.
A1 ATP and 2NADPH2B2 ATP and 2NADPH2C3 ATP and 2NADPH2D2 ATP and 1NADPH2The ratio of number of σ -bond to π− bond in N2 and CO2 molecules are
A2.0,2.0B2,12C12,1D12,2
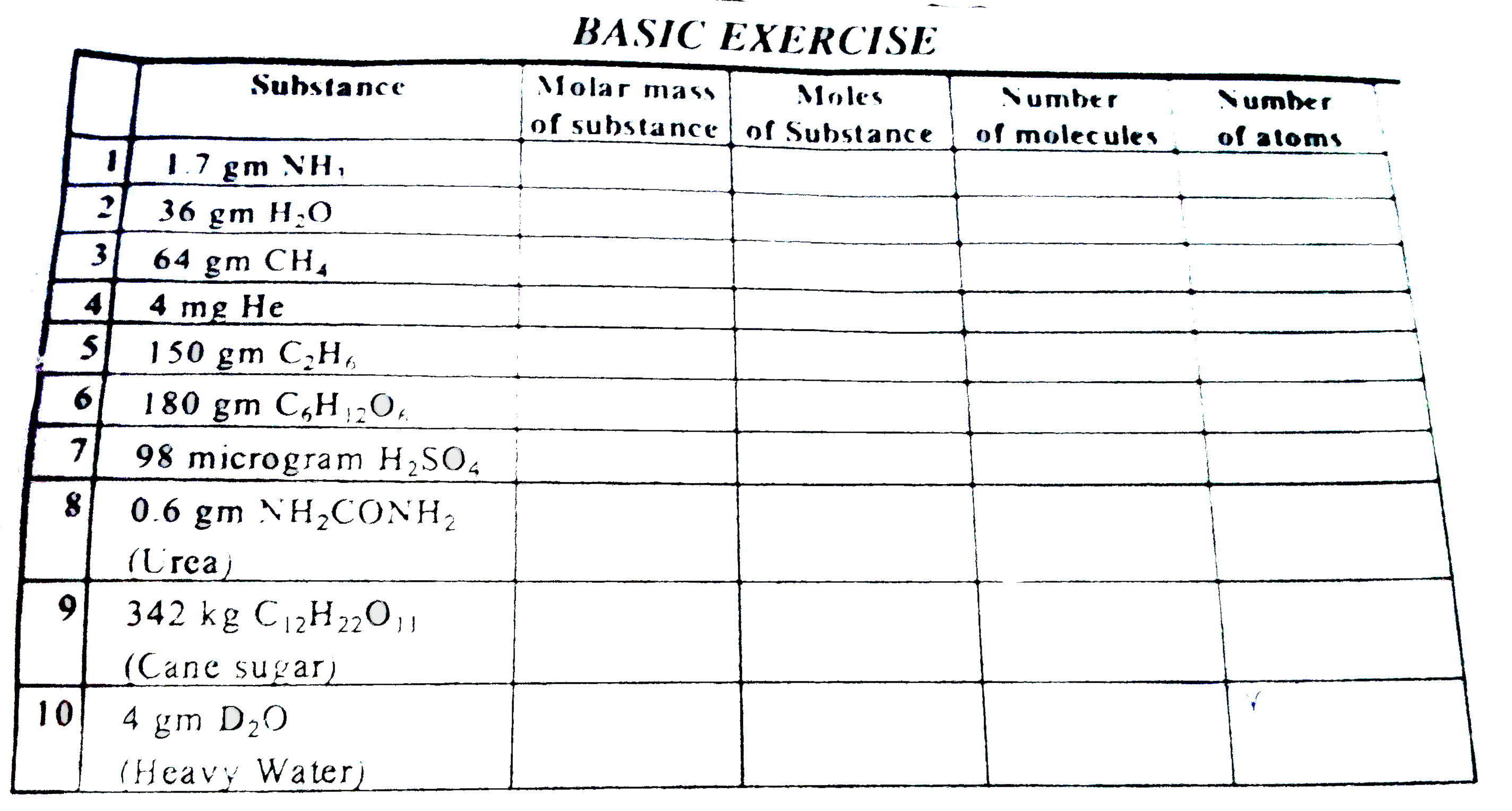
What is the mass of one CO2 molecules in gm?
View SolutionAssertion :-CO2 molecules is non-polar while SO2
Reason : - Carbon atom is smaller than sulphur.
View SolutionIn C3 cycle for the fixation of every CO2 molecule, the reduction and regeneration steps require :
View SolutionIf 15 Acetyl CoA enter in to Krebs cycle then how many CO2 molecules are produced after complete decarboxylation:
View SolutionWhen 12 CO2 molecules are utilized in C3 cycle, number of triose phosphates exported out from the chloroplast into the cystosol for the synthesis of hexose will be
View Solution