Which of the following molecules has the shortest carbon-carbon bond ?
A
(1) Acetylene
B
(2) Ethane
C
(3) Benzene
D
(4) Diamond
Video Solution
Text Solution
The correct Answer is:A
The decreasing order of bond length is
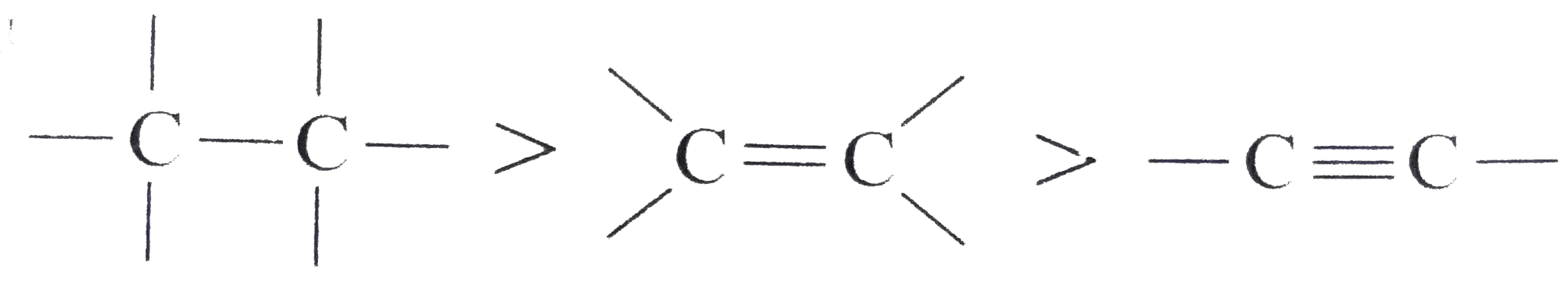
Acetylene has triple bond, benzene has double character, while both ethane and diamond have C−C single bonds.
|
Similar Questions
Knowledge Check
Which of the following molecules has the shortest carbon-carbon single bond length ?
ACH2=CH−C−CHBCH2=CH−C≡NCCH2=CH−CH−ODCH2=CH−CH=CH2Which of the following molecules has the shortest carbon single bond length ?
ACH2=CH−O||C−OHBCH2=CH−C≡NCCH2=CH−CH=ODCH2=CH−CH=CH2Which of the following compounds the shortest carbon-carbon bond length?
ABenzeneBEtheneCEthyneDEthane
Which one of the following has the shortest carbon carbon bond length?
View SolutionWhich of the following molecules has the highest value of carbon-carbon bond energy?
View SolutionWhich of the following molecules contains the shortest C−H bonds?
View SolutionWhich of the following has the shortest bond length
View SolutionWhich of the following has shortest carbon-carbon bond length?
View Solution

