The CH3− group in propene exerts
Hyperconjugation
+I effect
−I effect
both (1) and (2)
Inductive effect
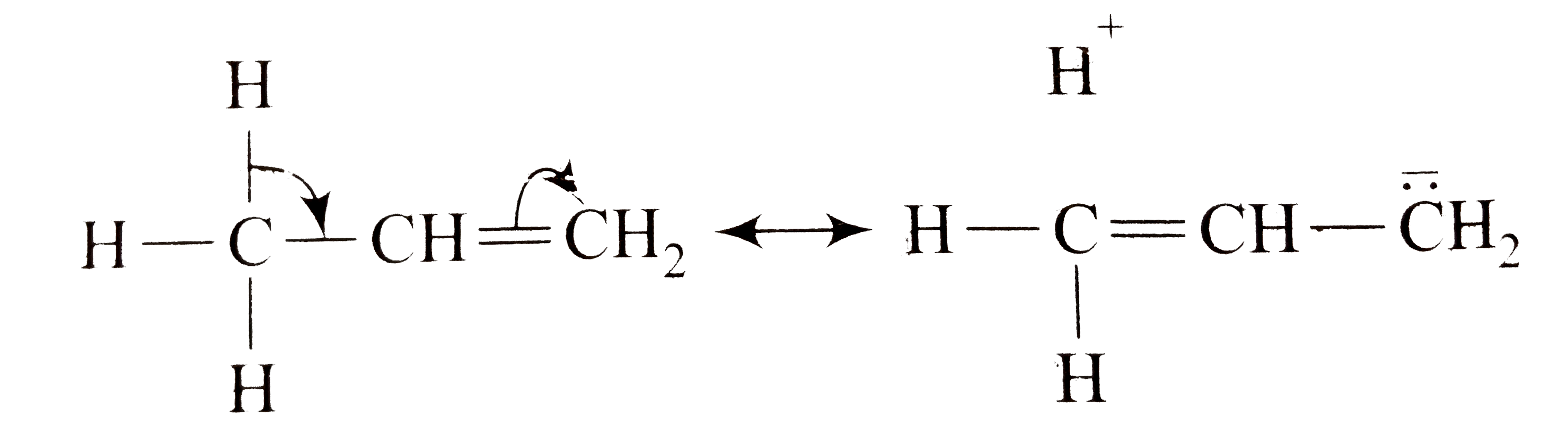
Similar Questions
Knowledge Check
Assertion : p-Nitrophenol is a stronger acid than p-cresol.
Reason : NO2 group is an electron withdrawing group while CH3 group is electron releasing in nature.AIf both assertion and reason are correct and reason is correct explanation for assertion.BIf both assertion and reason are correct but reason is not correct explanation for assertion.CIf assertion is correct but reason is incorrect.DIf assertion and reason both are incorrect.The (CH3)3C- group in t-butylacetylene exerts
A−I effectB+I effectChyperconjugationDboth (2) and (3)Assertion. Propene is more reactive than ethene towards electrophilic addition reactions
Reason. Hyperconjugation effect of the CH3 group increases the electron density in the double bondAIf both assertion and reason are true, and reason is the true explanation of the assertion.BIf both assertion and reason are true, but reason is not the true explanation of the assertion.CIf assertion is true, but reason is false.DIf both assertion and reason are false
(a) Match the formulae in group A with appropriate names from groupB:
Group A: CH3COOH,CH3CHO,CH3OH
Group B: Ethanol, Methanol, Ethanal, Ethanoic acid
View SolutionStatement 1: Acetoacetic ester (CH3COCH2COOC2H5) contains CH3CO group but does not give iodoform test.
Statement-2: The H-atoms of the CH3 group are more acidic than those of CH2 group.
View SolutionAssertion: Acetoacetic ester (CH3COCH2COOHH5) contains CH3CO group but does not give idoform test.
Reason : The H-atoms of the CH3 group are more acidic than those of CH2 group
View SolutionSTATEMENT - 1 : OH OH CH3 O | | | |∣CH3CH2−C −C−CH2CH3H⊕−−→△CH3CH2 −C −C−CH3 | | | | CH3 CH3 CH3CH2 CH3 (Major products)
STATEMENT - 2 : CH3CH2group shifts during this reaction as it is a better electron donor so better migrator than -CH3group.
View SolutionA: CH3CH=CH2 can undergo cationic polymeisation with greater ease than CH2=CH2.
R: CH3− groups has +I effect.
View Solution
