Which of the following is a free-radical substitution reaction?
A
CH3CH2CH2Br+KOHΔ−−→CH3CH2CH2OH
B
CH3CH=CH2+Cl2heat−−→CH2=CH−CH2CI
C
CH2=CH2+Br2→BrCH2CH2Br
D
Video Solution
Text Solution
The correct Answer is:B
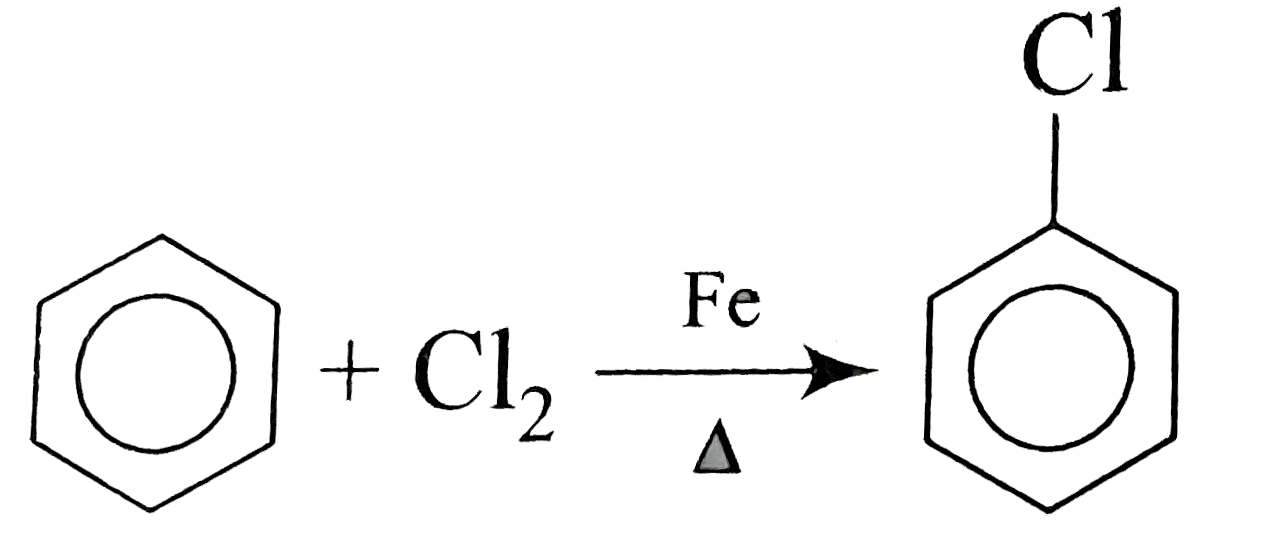
It is an example of allyic substitution initiated by chlorine free radicals. Reaction (1) is nucleophilic displacement , reaction (3) is electrphilic addtion , and reaction (4) is electrphilic aromatic substitution
|
Similar Questions
Knowledge Check
Which of the following is a free radical susbtitution reaction
A B
B C
C D
D
Which of the following is a free radical susbtitution reaction ?
ABenzene + Me−ClAnhyd.−−−→AlCl3Ph−CH3B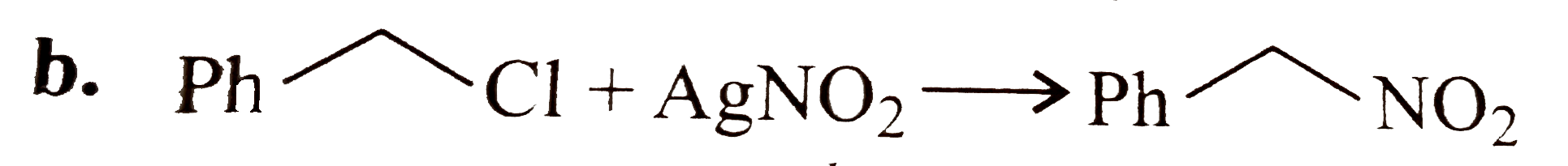 C
C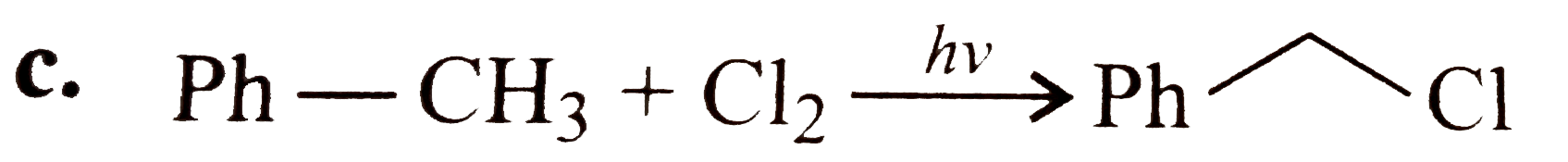 D
D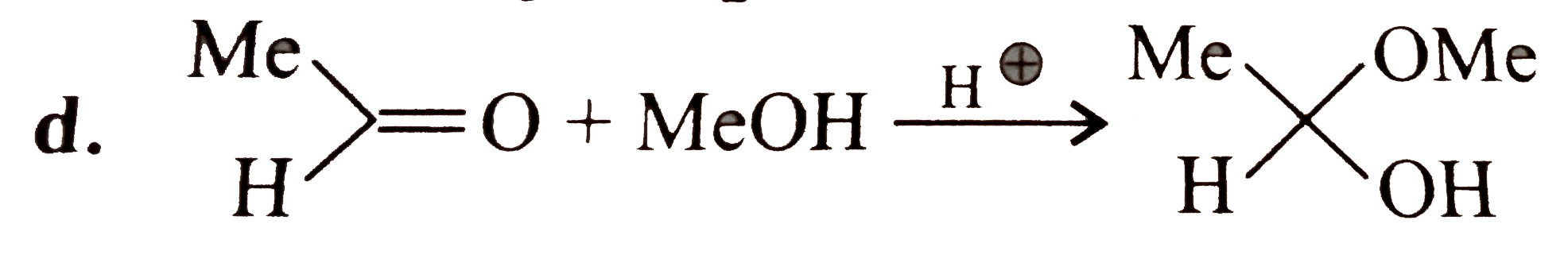
Which one of the following is a free-radical substitution reaction ?
A B
B C
C DCH3CHO+HCN→CH3CH(OH)CN
DCH3CHO+HCN→CH3CH(OH)CN
Which one of the following is a free - radical substitution reaction ?
View SolutionWhich one of the following is a free - radical substitution reaction ?
View SolutionWhich one of the following is a free-redical substitution reaction?
View SolutionWhich of the following is free radical?
View SolutionWhich of the following is not a free radical ?
View Solution

