Which of the following statements is not correct about the resonance strucuture?
A
Contributing structures are less stable than the resonance hybrid.
B
Contributing structures represent molecules having no real existence.
C
Equivalent resonating structure make the resonance very important.
D
Contributing structure contribute to the resonance hybrid in proportion of their relative energies.
Video Solution
Text Solution
The correct Answer is:D
Relative contribution of a resonance structure is inversly proportional its energy but directly proportional to its stability. Thus, the lesser the energy and the more stable the sturcutre, the greater is its contribution to the hybrid.
|
Similar Questions
Knowledge Check
Which of the following statement is correct about bile?
AIt helps in emulsification of fat.BIt helps in digestion of carbohydratesCIt helps in absorption of digested food.DIt helps in egestion of undigested food.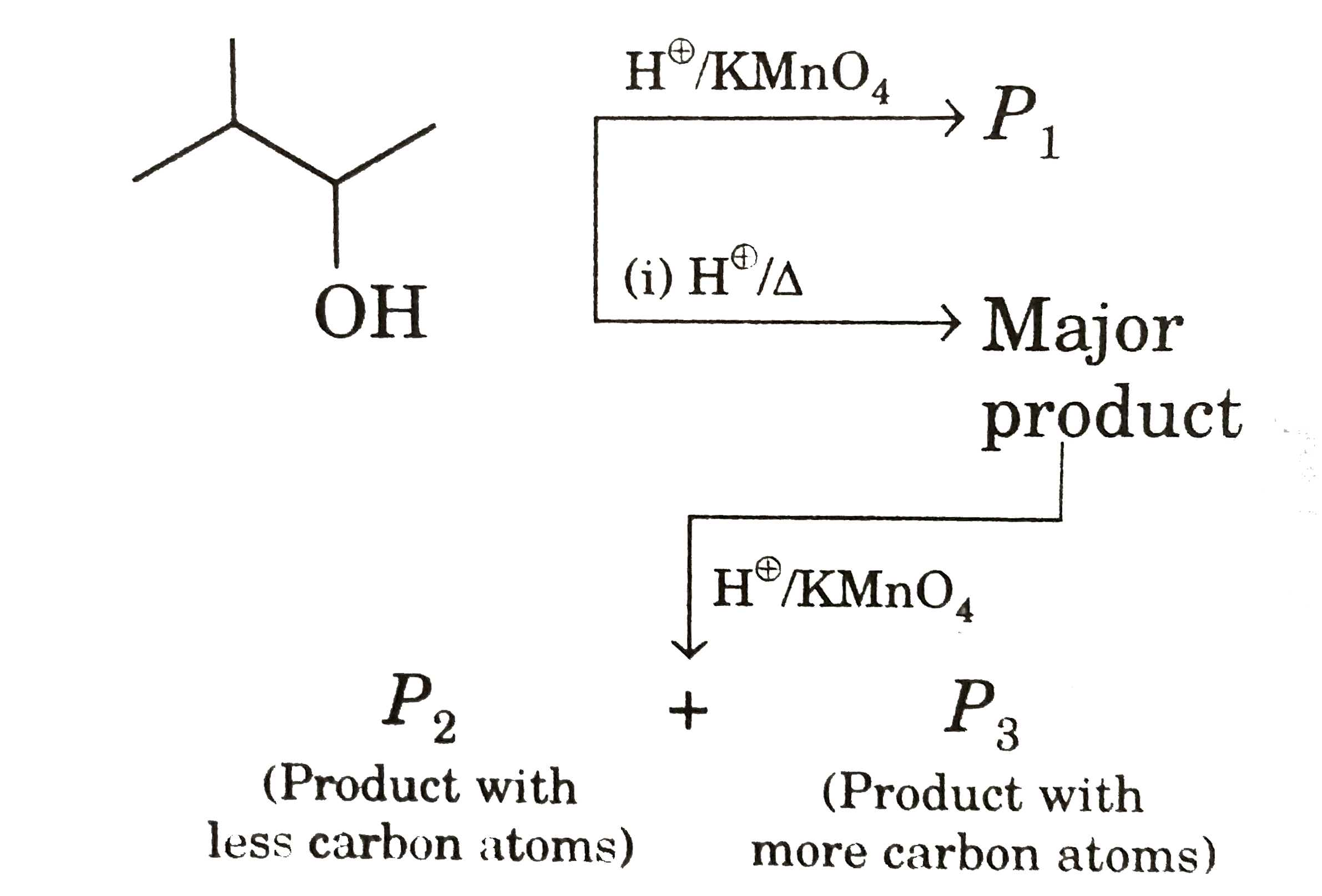
Which of the following statement is not correct about P2 ?AIt will give non-resolvable product with MeMgBrBIt can not give effervescence of CO2 with NaHCO3CIt gives alkane on sodalime decarboxylationDIt can change blue litmus into redWhich of the following statement is not correct about dinitrogen.
AN2 is a diatomic molecule.BHydrogen bonding is absent in NH3CNitrogen shows covalency of 3.DNitrogen is chemically inert.
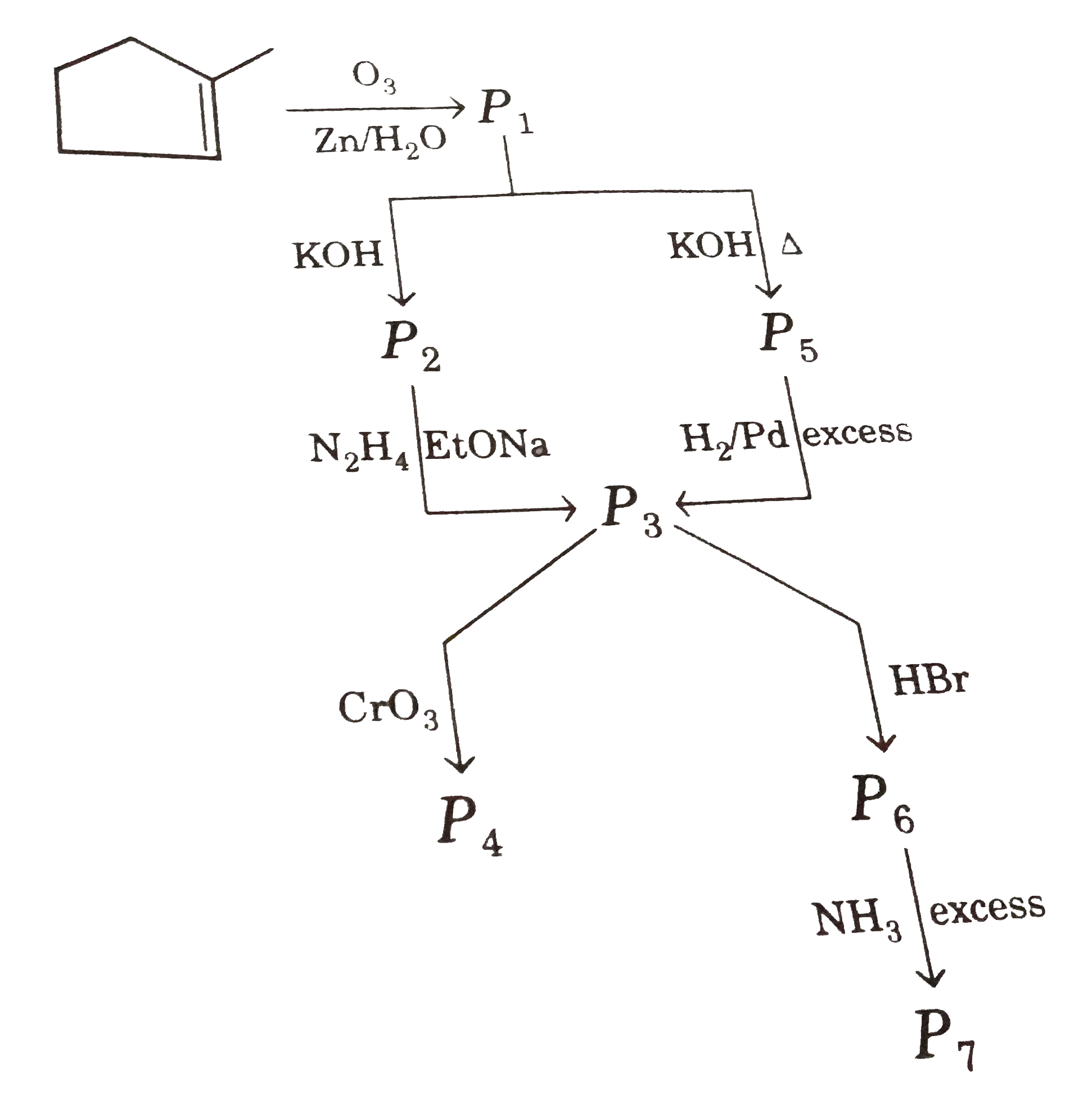
Which of the following statement is correct about P4 ?
View Solution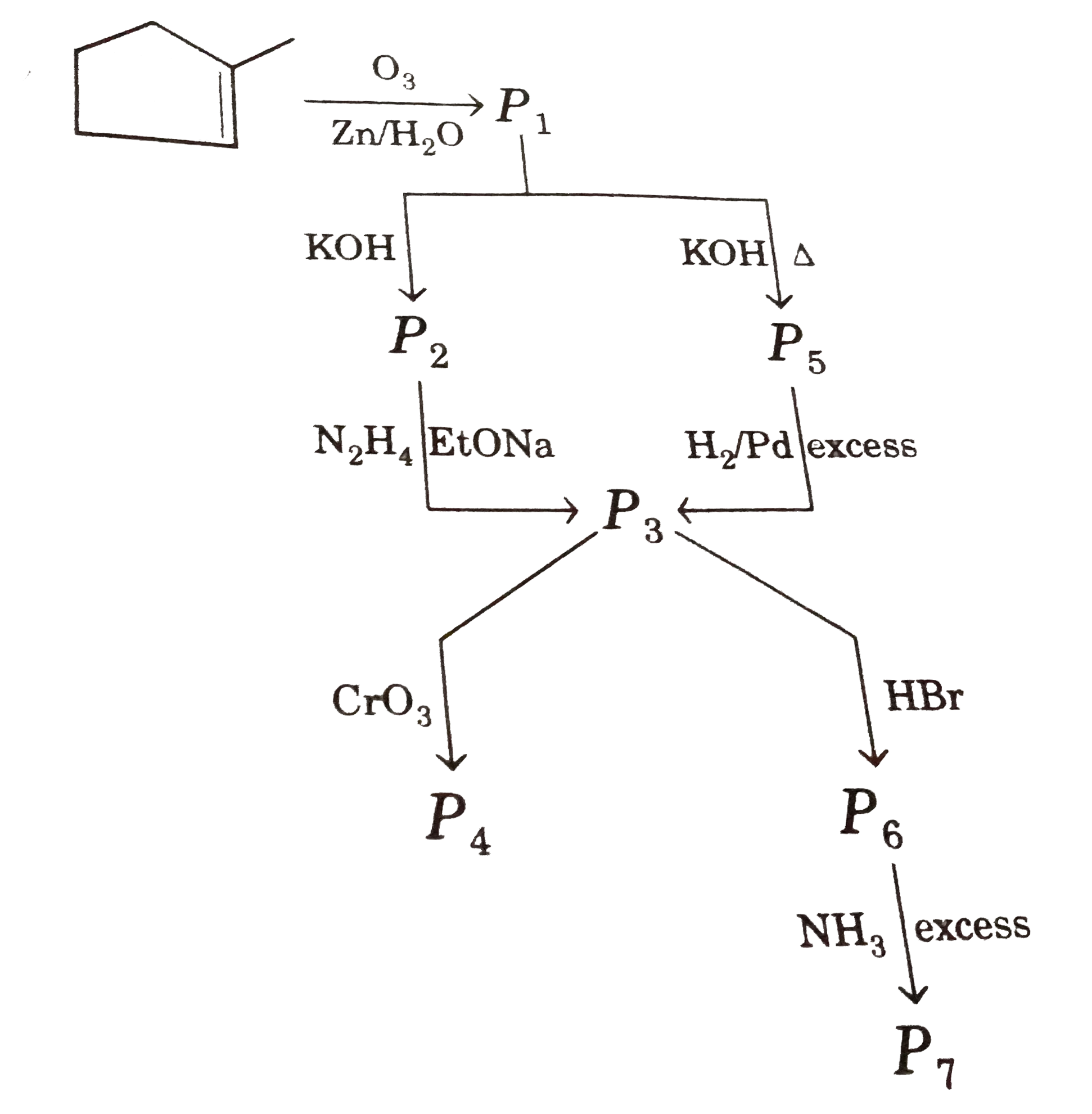
Which of the following statement is correct about P7 ?
View SolutionWhich of the following statement is correct about alkanes?
View SolutionWhich of the following statements is not correct about hemichordata?
View SolutionWhich of the following statement is not correct about group-16?
View Solution

