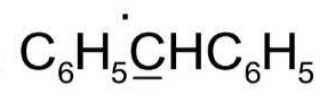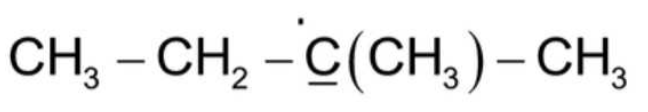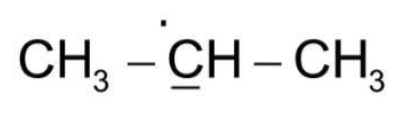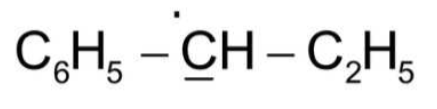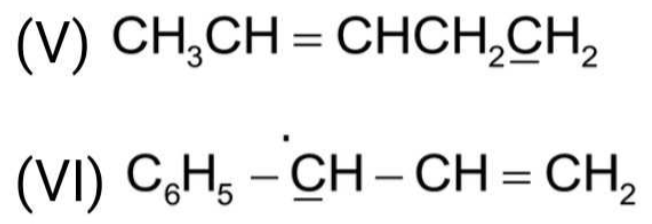Which of the following free radicals is the most stable?
A
.CH3
B
.CH2CH2CH(CH3)2
C
CH3.CHCH2CH3
D
CH3CH2.C(CH3)2
Video Solution
Text Solution
The correct Answer is:D
Order of stabilityd of free radicals
Tertiary gt Secondary gt Primary gt Methyl
Radicals are electron-dificient because C has only 7 electrons in its valence shell. Since R groups are electron-donating via induction, the more alkyl groups bonded to the electron deficient C, the more stable the radical is.
|
Similar Questions
Knowledge Check
Which of the following free radicals is most stable?
APrimaryBMethylCSecondaryDTertiaryWhich among the following free radicals is most stable
A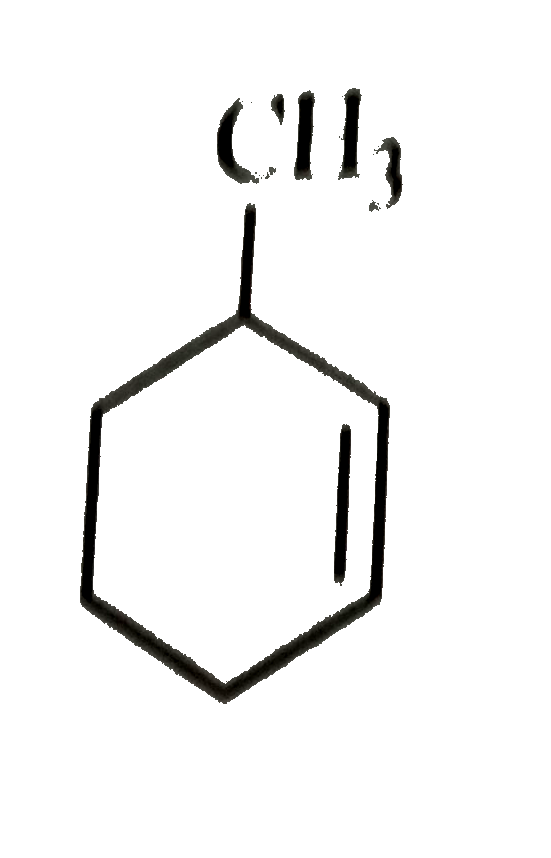 B
B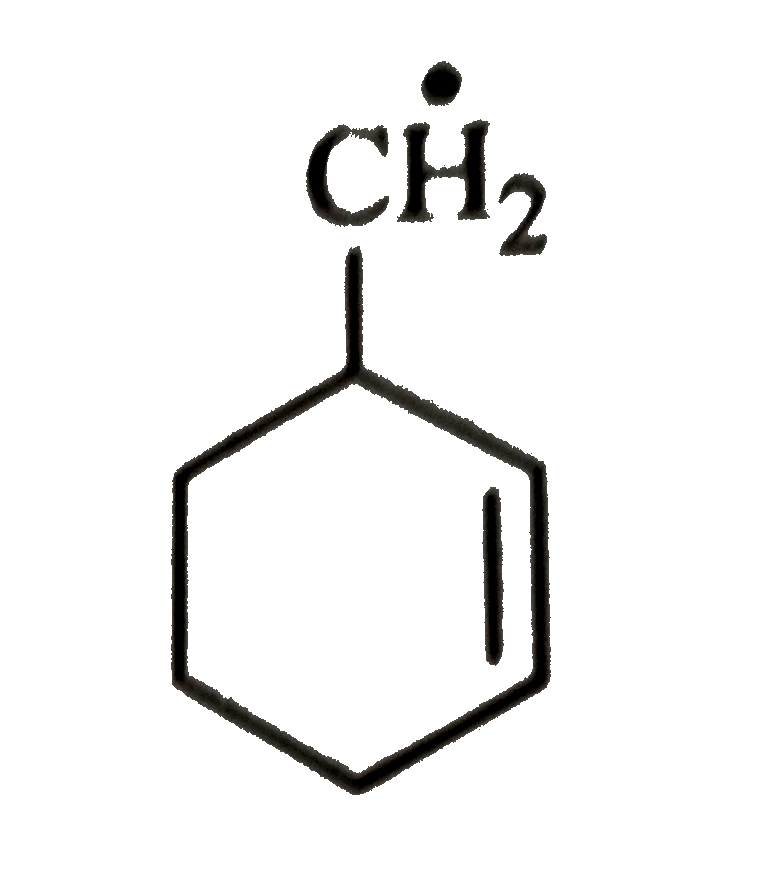 CC.H3D
CC.H3D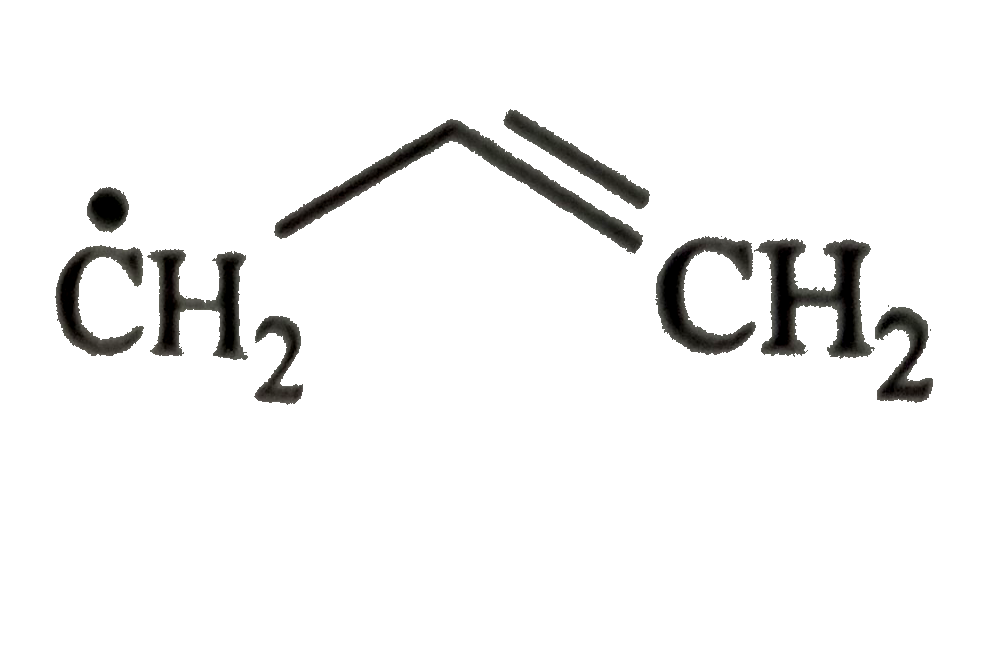
Which of the following is free radical?
ANO2BCl−CClDCl+
Which free radical is the least stable ?
View SolutionWhich of the following carbonates is the most stable
View SolutionWhich of the following is not a free radical ?
View SolutionWhich of the following free radicals would be expected to be most selective?
View SolutionMaximum stability will be in which of the following free radicals?
View Solution