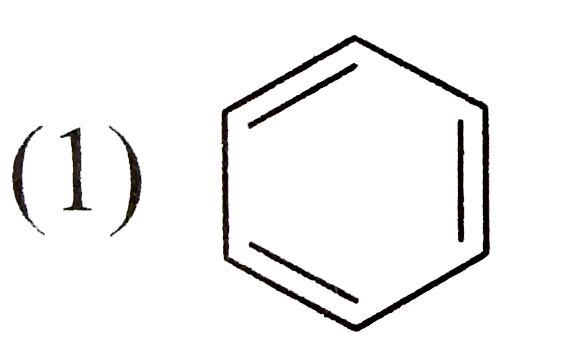
Which of the followin is the resonance hybrid of benzene?
A
B
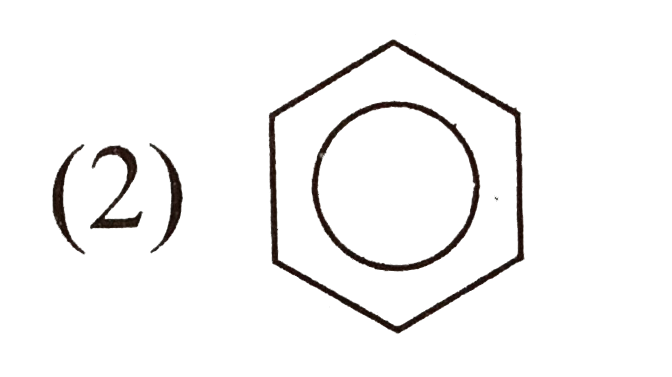
C
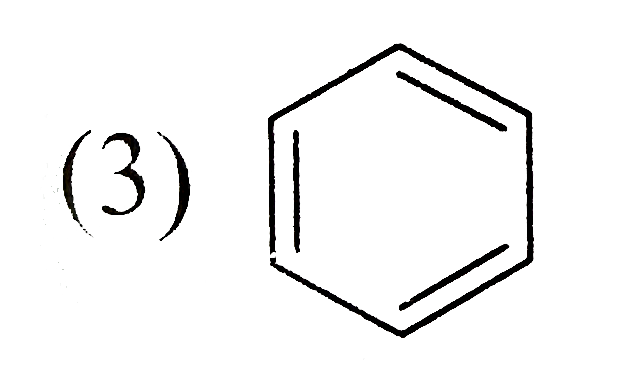
D
Both (1) and (3)
Video Solution
Text Solution
The correct Answer is:B
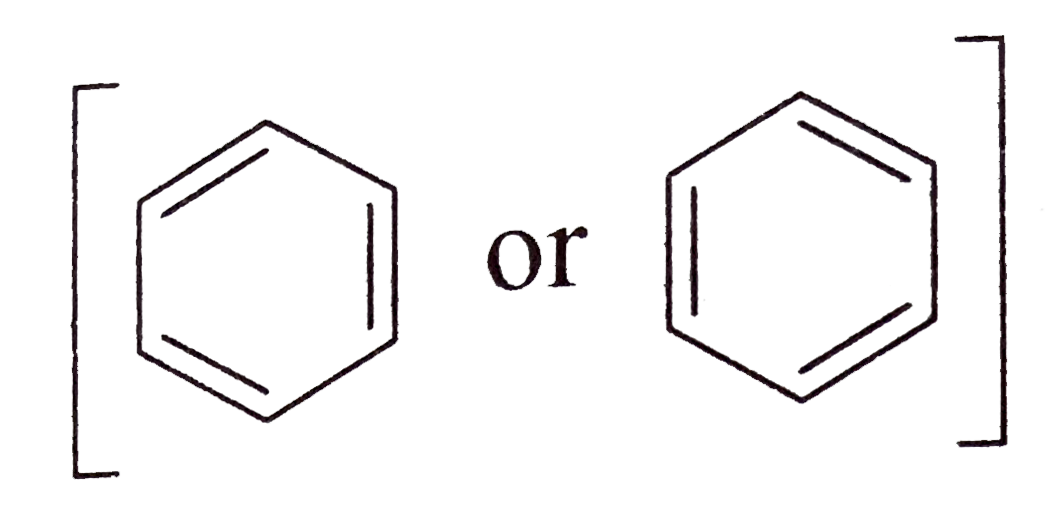
Structures (1) and (3) are the kekule resonance structures. The resonance hybrid has only one C- to -C bond length and bond energy. This length is intermediate between that of a single and double bond. The hypothetical cyclohexatriene or would have two different alternating C-C and C=C bond length with different bond energies The hybrid is more stable and hence less reactive than the hypothetical cyclohexatriene.
|
Similar Questions
Knowledge Check
Aniline is a resonance hybrid of
A3 structuresB6 structuresC2 structuresD5 structuresThe resonance energy of benzene is
A36kcal/molB85.8kJ/moleC150.48kJ/moleDBoth 1 & 3Resonance hybrid of nitrate ion is
ABCD

