According to the huckel rule, planar and completely conjugated monocyclic polyene is aromatic if it possesses
(4n+1)π electrons
(4n+2)π electrons
(2n+2)π electrons
4nπ electrons
The correct Answer is:B
Huckel's rule deals with compounds containing one planar ring in which each atoms has a p orbital his calculation show that planear monocyclic rings containing (4n+2)π electrons, where n=0,1,2,3.. And so on have closed shells of delocalized e−s like benzene.
Similar Questions
Knowledge Check
According to Huckel rule a cyclic conjugated polyene is aromatic if it constants :
A(4n+1)π - electronB(4n+2)π-electronC(2n+2)π-electronD4nπ- elecrtonStatement-I:
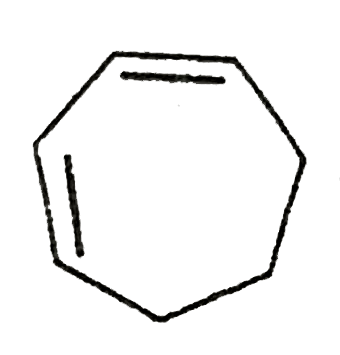 is an anti-aromatic compound.
is an anti-aromatic compound.
Statement-II: Cyclic, planar, completely conjugated species having 4n π electrons are anti-aromatic.AIf both statement-I & statement-II are true & the statement-II is a correct explanantion of the statement-IBIf both statement-I & statement-II are true but statement-II is not a correct explanation of the statement-ICIf statement-I is true but the sttement-II is false.DIf statement-I is false but the statement -II is true.According to Huckel, monocyclic compounds will show aromaticity when it has
A4π electronsB6π electronsC4π+2 electronsD(4n+2)π electrons
According to (n+l) rule after completing 'np' level the electron enters into
View SolutionAccording to (n+l) rule after completing 'np' level the electron enters int
View SolutionFor any compound to be aromatic, compound should follow a given set of rule known as Huckel's rule
According to Huckel's rule of aromaticy :
(a) compound should be cyclic
(b) compounds shoulds be planar and conjugated .
(c) compound should have (4n+2)πe−
where n=0, 1, 2, 3.... integer number .
Which of the following is not an aromatic compound ?
View SolutionFor any compound to be aromatic, compound should follow a given set of rule known as Huckel's rule
According to Huckel's rule of aromaticy :
(a) compound should be cyclic
(b) compounds shoulds be planar and conjugated .
(c) compound should have (4n+2)πe−
where n=0, 1, 2, 3.... integer number .
Among the following which is a non-planer compound ?
View SolutionFor any compound to be aromatic, compound should follow a given set of rule known as Huckel's rule
According to Huckel's rule of aromaticy :
(a) compound should be cyclic
(b) compounds shoulds be planar and conjugated .
(c) compound should have (4n+2)πe−
where n=0, 1, 2, 3.... "integer number" .
"Identify the compound which have maximum dipole moment" :
View Solution
